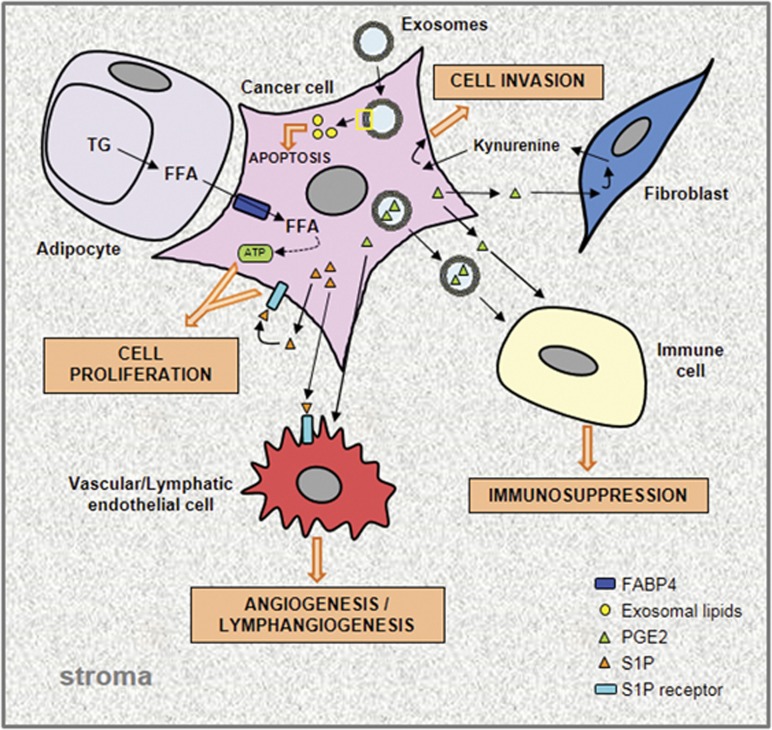
Figure 3.
Tumor–stroma bidirectional dialog. Schematic representation of lipid exchanges between cancer cells and the different cell types found in the TME. In adipocytes adjacent to cancer cells, the hydrolysis of TG, stored in LDs, releases free fatty acids (FFAs) which are taken up by cancer cells, transported through fatty acid binding protein 4 (FABP4) and degraded to provide ATP needed for their growth. Bioactive lipids secreted by cancer cells, PGE2 and S1P, exert their effects on stromal cells through paracrine mechanisms. The PGE2, transported or not by exosomes, promotes angiogenesis and also immunosuppression. The latter effect results from an activation of myeloid-derived suppressor cells and differentiation of monocytes into suppressor macrophages. Moreover, tumor-derived PGE2 induces kynurenine secretion by CAFs which in turn promote cancer cell invasiveness. S1P, by its binding on its specific receptor, promotes cancer cell proliferation and angiogenesis/lymphangiogenesis in an autocrine and paracrine manner, respectively. Taken together, FFA and free bioactive lipids contribute toward promoting tumor growth. Exosomes in TME contain high lipid levels within the membrane and lumen, and therefore constitute extracellular lipid sources which can be internalized by cancer cells and are responsible for the increased cell lipid concentration which triggers an ERS-induced cell death.