Figure 2.
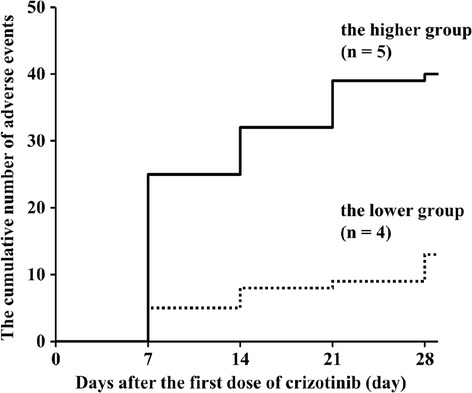
The cumulative numbers of adverse events in the higher and in the lower crizotinib trough concentration groups. The crizotinib trough concentration is divided into a higher trough concentration group (n = 5, −−−−−−−) and a lower trough concentration group (n = 4, −------) delimited at the median concentration (508.5 ng/mL). Adverse events were evaluated four times (day 7, 14, 21, and 28).
