Figure 1.
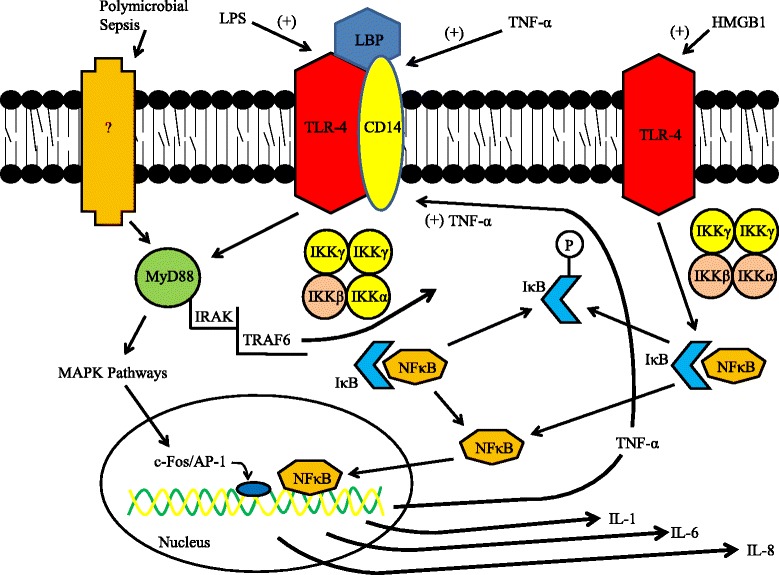
Schematic illustration of the activation of the NF-κB transcription factor pathway by liposaccharide (LPS), TNF-α, high mobility group (HMG)B1, and polymicrobial sepsis. LPS binding to Toll-like receptor-4 (TLR-4) is dependent on its interaction with circulating LPS-binding protein (LBP) and the subsequent interplay of this complex to membrane bound CD14 [90]. This LPS-LBP-CD14-TLR-4 complex is thus anchored to various cells within the kidney (especially within the apical brush border of proximal tubules) [60], where it initiates intracellular signaling cascades. CD14 gene expression is upregulated by LPS [91] in human kidney proximal tubular epithelial cells and by TNF-α [92] in mouse renal interstitial and tubular epithelial cells. Activated TLR-4 via LPS stimulates the MyD88 pathway [47,93,94], and ultimately induces phosphorylation of IκB by IκB-kinase-β (IKKβ; tan-colored) within the IKK complex [65]. Phosphorylated IκB is then released from NF-κB, allowing NF-κB to migrate to the nucleus to modulate transcription of various cytokines as shown. HMGB1 activation of TLR-4 activates NF-κB via an alternative mechanism whereby both IKKα and IKKβ (tan-colored) are implicated [54,55]. In cecal ligation and puncture models of sepsis, MyD88 has been shown to be activated independent of TLR-4 by unknown upstream cell surface receptors [72,75]. IRAK, interleukin receptor-associated kinase; MAPK, p38 mitogen-activated protein kinase; TRAF, TNF receptor associated factor.
