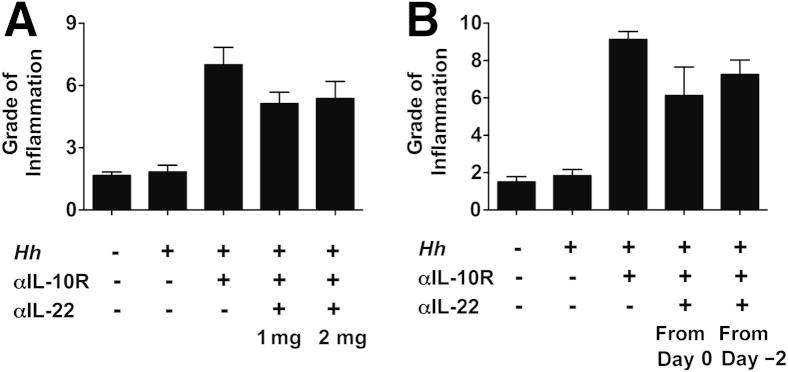
Figure 3.
Increasing the dose of anti–IL-22 or starting monoclonal antibody (mAb) treatment before Helicobacter hepaticus (Hh)/anti–IL-10 receptor (IL-10R) inoculation does not affect cecal pathology. C57BL/6 wild-type mice were inoculated with H. hepaticus and treated with anti–IL-10R plus anti–IL-22 as indicated. One week after the last mAb administration, pathology was analyzed in the cecum. Uninfected mice were included as controls. A: A total of 1 or 2 mg of anti–IL-22 was given on days 0 and 7 postinfection (pi). B: A total of 1 mg of anti–IL-22 was given on days 0 and 7 pi or on days −2, 0, and 7 pi. Data represent grade of inflammation (using a scoring system on the basis of five parameters of inflammation: epithelial hyperplasia, lamina propria cellularity, goblet cell depletion, crypt abscesses, and ulcers). Data are means ± SEM (A and B). n = 4 mice per group (A and B, H. hepaticus/anti–IL-10R, H. hepaticus/anti–IL-10R/anti–IL-22 1 mg, and H. hepaticus/anti–IL-10R/anti–IL-22 2 mg; and B, uninfected mice); n = 3 mice per group (A and B, H. hepaticus alone; and A, uninfected mice).