Fig. 4. Gel electrophoretic pattern and activity of 125I-IL-6.
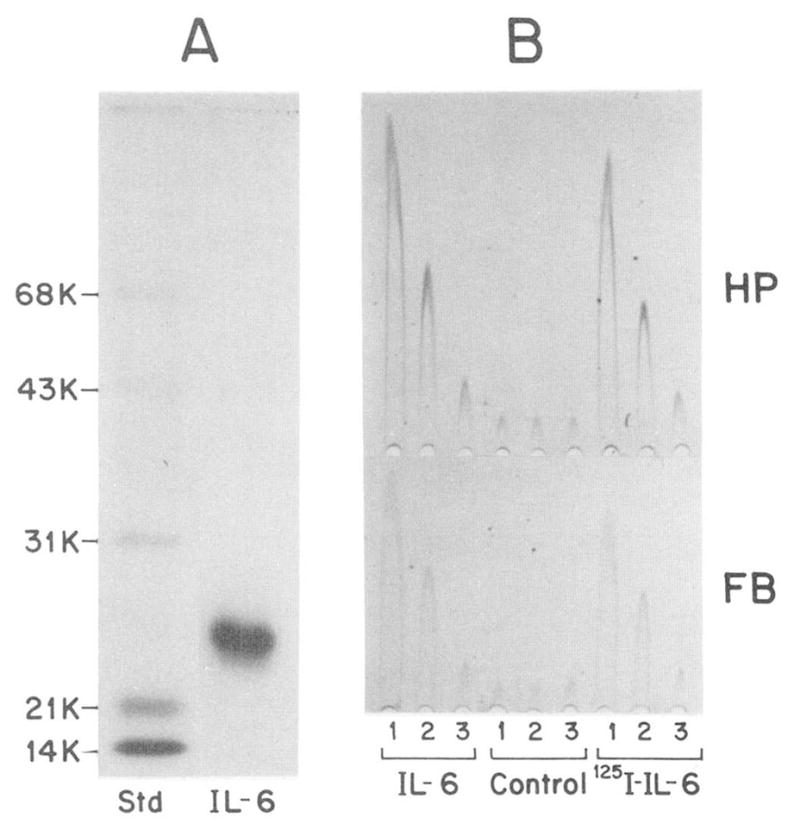
In A, an aliquot of IL-6 labeled with the 125I-Bolton Hunger reagent (65,000 cpm) was separated together with molecular weight standard (Std) on an 11% sodium dodecyl sulfate polyacrylamide gel. The fluorogram shown was exposed for 3 h. (The 125I-IL-6 protein band at Mr 24,000 yielded 59,800 cpm.) In B, medium (MEM, 1% fetal calf serum, 1 μM dexamethasone) containing 10, 1, or 0.1 ng/ml of unlabeled IL-6 or 125I-IL-6 (lanes 1, 2, and 3, respectively), was tested on HepG2 cells. The stimulated production of haptoglobin (HP) and fibrinogen (FB) was measured by rocket Immunoelectrophoresis (20). Control represents HepG2 cells treated with medium containing the buffer used for chromatography of 125I-IL-6 (50 mM sodium phosphate, pH 7.5, 0.25% gelatin; Ref. 41). The amount of buffer added was the same as used in the experimental assays.
