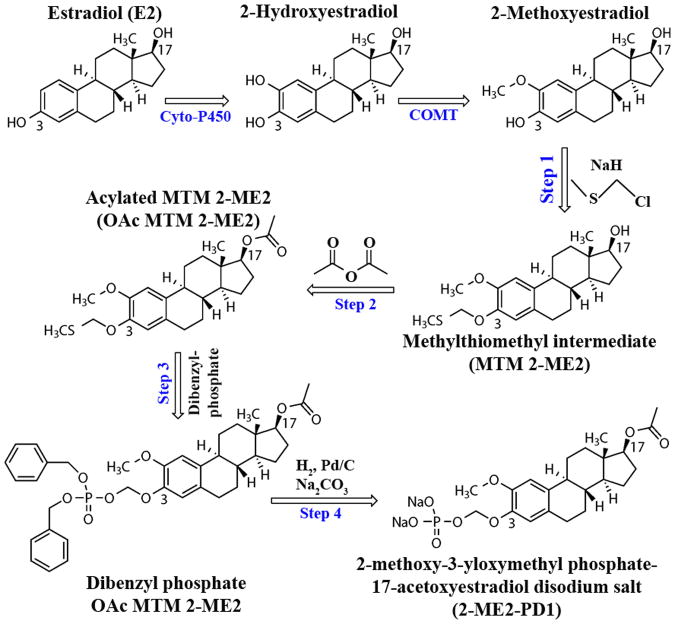
Figure 1. Sequential events of synthesis of the 2-ME2 prodrug, 2-ME2-PD1, from 2-Methoxyestradiol.
The first step in the synthesis of 2-ME2-PD1 is the selective formation of the methylthiomethyl ether intermediate at the phenolic hydroxyl of 2-ME2 (selectively favored over the secondary alcohol on the opposite side of the molecule). This step was followed by acylation of the secondary alcohol (to protect and hinder metabolism at C-17 position). The acetylated product was purified by column chromatography and used in the formation of the protected phosphate ester. Final removal of the dibenzyl groups on the phosphate ester (C-3 position) and formation of the sodium salt gives the desired prodrug structure.