Abstract
CYP2D6 is primarily responsible for the metabolism of clomiphene citrate (CC). The purpose of the present study was to investigate the relationship between CYP2D6 genotypes, concentrations of CC and its major metabolites and drug response in infertility patients. We studied 42 patients with ovulatory dysfunction treated with only CC. Patients received a dose of 100 mg/day CC on days 3-7 of the menstrual cycle. CYP2D6 genotyping and measurement of CC and the major metabolite concentrations were performed. Patients were categorized into CC responders or non-responders according to one cycle response for the ovulation. Thirty-two patients were CC responders and 10 patients were non-responders with 1 cycle treatment. The CC concentrations were highly variable within the same group, but non-responders revealed significantly lower (E)-clomiphene concentration and a trend of decreased concentrations of active metabolites compared to the responders. Nine patients with intermediate metabolizer phenotype were all responders. We confirmed that the CC and the metabolite concentrations were different according to the ovulation status. However, our results do not provide evidence for the contribution of CYP2D6 polymorphism to either drug response or CC concentrations.
Keywords: Clomiphene, Ovulation Induction, Cytochrome P-450 CYP2D6
Graphical Abstract
INTRODUCTION
Clomiphene citrate (CC) is used for inducing ovulation in patients with polycystic ovarian syndrome (PCOS) or ovulatory dysfunction without evident cause (1,2). It is a structurally heteromorphic molecule with combination of zuclomiphene [(Z)-clomiphene] and enclomiphene [(E)-clomiphene], of which the (E)-clomiphene is more active in inducing ovulation (3,4). The in vivo metabolism of CC has not been revealed much over the years; however, 9 metabolites were recently discovered (4,5). CYP2D6 was reported to be involved in the metabolism of CC in a few studies (4,6) and CYP2D6 polymorphism could affect the production of active metabolites. Until now, there was no study on relationship between CYP2D6 genotypes and clinical drug effect or serum levels of active metabolites in infertile women taking CC. One recent study reported that the concentrations of (E)-4-OH-Clom and (E)-4-OH-DE-Clom were 8 to 12 times lower in healthy women volunteers with poor metabolizer (PM) compared to those with extensive metabolizer (EM) (4).
The purpose of this study was to investigate the relationship between CYP2D6 genotypes and CC metabolite concentration as well as the clinical drug response in patients with ovulatory dysfunction. Because CC has only minor adverse effects (7), the drug response was determined as the presence of ovulation after 1 cycle of CC treatment. The hypothesis of this study is as follows. Compared to patients with EM, those with intermediate metabolizer (IM) or PM would be predicted to have lower active metabolite concentrations. In addition, the possibility of decreased drug effects were expected to be greater, which means more frequent resistance to CC, in patients with lower metabolic CYP2D6 activity such as PM or IM.
MATERIALS AND METHODS
Subjects
A total of 42 patients with ovulatory dysfunction participated in this study between February 2011 and January 2013. None of the patients took other medications at the beginning of the study. Study participants were considered healthy based on medical history, physical examination and routine biochemical laboratory test results. Patients who had other infertility factors were excluded. All patients were ethnically Korean.
Study design
Patients consecutively received a dose of 100 mg/day CC on days 3-7 of the menstrual cycle, and blood samples were collected on days 3 and 10 (or on follow-up days). Serum specimens were used for the measurement of CC and its metabolites, anti-Müllerian hormone (AMH), follicle stimulating hormone (FSH), and estradiol (E2). Whole blood samples were prepared for CYP2D6 genotyping. Patients were monitored for follicular growth 2 to 3 times after the medication, and the diameter of dominant follicle was measured. Ovulation was assumed to have occurred if the diameter of dominant follicle was greater than 18 mm during the serial sonographic examination. In such a case, the presence of luteinizing hormone surge was confirmed by urine test. Patients who ovulated on an initial dose of 100 mg/day were regarded as CC responders, and those who did not ovulate on this dose were regarded as CC non-responders with 1 cycle treatment. For each group, the functional ovarian reserve was assessed by AMH and clomiphene citrate challenge test.
CYP2D6 genotyping and determination of expected phenotypes
Genomic DNA was extracted from whole blood using a Qiagen DNA extraction kit (Qiagen, Hilden, Germany). For CYP2D6 genotyping, we performed polymerase chain reaction (PCR)-direct sequencing and multiplex long-PCR (8,9). We included primers to amplify most CYP2D6 variants detected in previous studies of Korean population (10,11). The predicted enzyme activity was determined based on previous references (12,13). Phenotypes are determined by the combinations of functional allele, decreased-function allele, and non-functional allele.
Measurement of clomiphene and its metabolites
Measurements of CC and its metabolites were performed with use of HPLC-tandem mass spectrometry (4,5). Quantification of CC includes both (E)-clomiphene and (Z)-clomiphene. Its metabolites, including (E)-4-OH-DE-Clom, (E)-4-OH-Clom, (E)-N,N-didesethylclomiphene, (E)-N-desethylclomiphene, (E)-clomiphene-N-oxide, (Z)-4-hydroxyclomiphene, (Z)-N,N-didesethylclomiphene, (Z)-N-desethylclomiphene, (Z)-clomiphene-N-oxide were also analyzed. Serum specimens collected on day 3 were considered to be basal sample and those collected on day 10 or follow-up days were post-medication sample. Measurement procedure of both sample pairs was performed in a batch.
Statistical analyses
Mann-Whitney test or Fisher's exact test were performed to analyze demographics and functional ovarian reserve of the patients. P value of less than 0.05 was considered to be statistically significant. SPSS 19.0 for Windows (SPSS Inc., Chicago, IL, USA) was used.
Ethics statement
This study was approved by the institutional review board of the Mizmedi Hospital (Approval No.: MIZMEDI-2011-01-01). Informed consent for genetic testing was received from all patients.
RESULTS
Clinical and laboratory characteristics of the patients
Patients' demographics and endocrine characteristics are presented (Table 1). There were 32 CC responders and 10 non-responders after one cycle of treatment. The proportion of PCOS was significantly higher in non-responder group and AMH was also higher in non-responder group (P=0.002 and 0.001, respectively). Ninety percent of non-responders (9/10) were PCOS patients.
Table 1. Clinical and endocrine characteristics of patients according to the response to clomiphene therapy.
| Characteristics | All patients* (n = 42) | CC responders* (n = 32) | CC non-responders* (n = 10) | P value† |
|---|---|---|---|---|
| No. of PCOS patients | 19 | 10 | 9 | 0.002 |
| Age, yr | 32.8 (4.7) | 33.1 (5.0) | 30.7 (9.0) | 0.067 |
| Weight, kg | 54.0 (11.3) | 53.0 (10.5) | 59.5 (16.0) | 0.424 |
| Height, cm | 162 (8.0) | 162 (8.0) | 162 (9.0) | 0.778 |
| BMI, kg/m2 | 20.4 (3.7) | 20.3 (3.6) | 21.9 (5.3) | 0.346 |
| AMH, ng/mL | 4.3 (4.5) | 3.0 (3.2) | 8.1 (7.9) | 0.001 |
| FSH, mIU/mL‡ | 11.3 (3.3) | 11.2 (3.2) | 12.1 (3.1) | 0.226 |
| E2, pg/mL§ | 39.0 (24.8) | 39.5 (28.8) | 33.5 (21.3) | 0.671 |
*Values are presented as median (interquartile range) except number of PCOS patients; †Mann-Whitney or Fisher's exact test was performed to compare two groups; ‡FSH values are the sum of measurements on day 3 and day 10; §E2 values are measured on day 3. CC, clomiphene citrate; PCOS, polycystic ovary syndrome; BMI, body mass index; AMH, anti-Müllerian hormone; FSH, follicle stimulating hormone, E2, estradiol.
The expected phenotypes were divided into 2 groups, EMs and IMs. There were 33 EM patients and 9 IM patients. AMH was lower in IM patients (4.6 ng/mL for EM and 2.5 ng/mL for IM, P=0.024), and this may be because there were only 2 PCOS patients in IM group while remaining 17 PCOS patients were in EM group. All IM patients were included in CC responder group and 30% of EM patients were CC non-responders.
CYP2D6 genotypes and determination of expected phenotypes
The major genotypes were CYP2D6*1/*10 (33.3%), *1/*2 (14.3%), *2/*10 (12.0%), and *10/*10 (9.5%). CYP2D6*10 was found to be the most frequent allele (41.7%), followed by CYP2D6*1 (34.5%). As previously mentioned, there were 33 EM and 9 IM patients. Genotypes of IM patients included CYP2D6*10/*10, *10/*41, *10/*49, and *5/*10 (Table 2). No PM or UM was detected.
Table 2. CYP2D6 genotypes and expected phenotypes.
| CYP2D6 genotype | Expected phenotype | No. of EM alleles | No. of PM alleles | Frequency | % |
|---|---|---|---|---|---|
| *1/*1 | EM/EM | 2 | 0 | 3 | 7.1 |
| *1/*2 | 6 | 14.3 | |||
| *2/*2 | 2 | 4.8 | |||
| *1/*10 | EM/IM | 1 | 0 | 14 | 33.3 |
| *1/*49 | 1 | 2.4 | |||
| *1/*52 | 1 | 2.4 | |||
| *2/*10 | 5 | 12.0 | |||
| *1/*5 | EM/PM | 1 | 1 | 1 | 2.4 |
| EM subtotal: 33 (78.6%) | |||||
| *10/*10 | IM/IM | 0 | 0 | 4 | 9.5 |
| *10/*41 | 1 | 2.4 | |||
| *10/*49 | 1 | 2.4 | |||
| *5/*10 | IM/PM | 0 | 1 | 3 | 7.1 |
| IM subtotal: 9 (21.4%) | |||||
| Total | 42 | 100.0 |
EM, extensive metabolizer; PM, poor metabolizer; IM, intermediate metabolizer.
Measurement of clomiphene and its metabolites
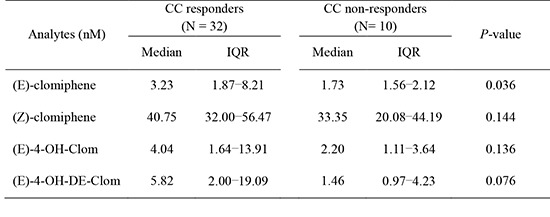
(E)- and (Z)-clomiphene, and other various metabolites were measured. In post-medication samples, CC and metabolites concentrations varied in large degrees. However, when we compared the results between CC responders and non-responders, (E)-clomiphene levels were significantly lower in non-responders, with showing similar trend in major active metabolites (Table 3). Although no statistical difference in the metabolites, there was a trend of showing lower levels in non-responders.
Table 3. Clomiphene and the metabolites concentrations in post-medication samples.
| Analytes (nM) | Total | CC responders (n=32) | CC non-responders (n=10) | P value* | |||
|---|---|---|---|---|---|---|---|
| Median | IQR† | Median | IQR† | Median | IQR† | ||
| (E)-clomiphene | 2.32 | 1.70-7.34 | 3.23 | 1.87-8.21 | 1.73 | 1.56-2.12 | 0.036 |
| (Z)-clomiphene | 38.44 | 29.06-52.93 | 40.75 | 32.00-56.47 | 33.35 | 20.08-44.19 | 0.144 |
| (E)-4-OH-Clom | 3.15 | 1.27-12.85 | 4.04 | 1.64-13.91 | 2.20 | 1.11-3.64 | 0.136 |
| (E)-4-OH-DE-Clom | 4.11 | 1.22-12.88 | 5.82 | 2.00-19.09 | 1.46 | 0.97-4.23 | 0.076 |
| (E)-N-desethylclomiphene | 0.64 | 0.30-4.33 | 1.13 | 0.38-5.73 | 0.50 | 0.18-0.87 | 0.173 |
| (E)-N, N-didesethylclomiphene | 4.73 | 1.13-10.99 | 5.21 | 1.29-11.36 | 2.96 | 0.97-12.60 | 0.637 |
| (E)-clomiphene-N-oxide | 0.00 | < LLOQ, 0.21 | 0.00 | < LLOQ, 0.23 | 0.00 | < LLOQ, 0.05 | 0.480 |
| (Z)-4-hydroxyclomiphene | 0.51 | 0.27- 1.05 | 0.58 | 0.31-1.45 | 0.33 | 0.25-0.71 | 0.199 |
| (Z)-N-desethylclomiphene | 13.36 | 10.28-20.01 | 12.33 | 10.21-19.03 | 15.53 | 10.31-20.19 | 0.516 |
| (Z)-N, N-didesethylclomiphene | 0.62 | 0.47-1.23 | 0.61 | 0.44-1.09 | 0.79 | 0.48-1.37 | 0.565 |
| (Z)-clomiphene-N-oxide | 8.70 | 1.30-18.09 | 7.26 | 0.84-17.27 | 16.49 | 10.29-18.98 | 0.048 |
*Mann-Whitney test was performed to compare two groups; †First and third quartiles are presented. CC, clomiphene citrate; IQR, interquartile range; LLOQ, lower limit of quantification; (E)-4-OH-Clom, (E)-4-hydroxyclomiphene; (E)-4-OH-DE-Clom, (E)-4-hydroxy-N-desethylclomiphene.
Among 42 patients, 4 patients had their samples taken on the first day after completing one cycle of CC treatment (day 8), 9 patients on the second day (day 9), 13 patients on the third day (day 10), 4 patients on the fourth day (day 11), 9 patients on the fifth day (day 12), and 3 patients on the sixth day (day 13). As the sample collection day was delayed, the concentration of the metabolites showed decreasing trend in patients. The timing of post-medication samples was determined by the ovulatory response of each patient. It was the day of ovarian response showing the peak serum levels of E2 in the stimulatory cycle with clomiphene in CC responders. It was also on the day of hCG triggering of the cycle. It was the last day of determined as CC non-responders with clomiphene stimulation. IM group had showed large differences despite of just 1 to 2 days of collection time point.
CC and some of its metabolites were observed in basal samples of several patients. (Z)-clomiphene was detected in 15 basal samples (median 4.29 nM). Of them, 9 patients had a previous history of CC medication within 1 to 14 months of sample collection. The (Z)-clomiphene concentration was relatively lower (0.41-2.27 nM) in 5 patients without any history of CC medication, but relatively higher (4.29-70.51 nM) in patients who had the history of CC medication just prior to study initiation within 1 to 3 months.
DISCUSSION
This is the first study on the relationship between concentrations of CC and the drug response in actual infertility patients. CC non-responders showed significantly lower (E)-clomiphene levels compared to responders. (E)-clomiphene is considered stronger isomer to produce ovulation induction (4,6,14). Decreased concentration of (E)-clomiphene may constitute the evidence for increasing the daily dose of CC in next cycle. Moreover, AMH level was significantly higher in non-responder group than responders, of which 90% were PCOS patients (9.3±4.6 vs. 3.8±2.6 ng/mL) in this study. Elevated serum AMH concentration in women with PCOS is known to lower sensitivity of ovarian follicles to circulating FSH. This effect may compromise the outcome of CC ovulation induction. Previous studies reported that PCOS women with high circulating AMH seem to be resistant to CC and may require a higher starting dose (15, 16). To evaluate prospectively the prognostic factors for ovulatory responses following CC administration in PCOS, endocrine and metabolic parameters between responder and non-responder groups were analyzed in the previous study (17). They had reported that the levels of blood glucose and blood glucose × insulin at 120 min after 75-g oral glucose tolerance test could be good biochemical markers of CC resistance in PCOS. Also the concentration of (Z)-clomiphene-N-oxide, one of the metabolites of (Z)-clomiphene, was significantly higher in non-responder group compared to responders. This might be one of the evidence for changing to other drugs (Table 3).
Another key point was in line with previous studies. In the post-medication samples, the concentration of (Z)-clomiphene was higher than that of (E)-clomiphene and (Z)-clomiphene was detected in substantial number of basal samples from those who were given CC before entering the study. This supports previous finding in which half-life of (Z)-clomiphene is much longer than (E)-clomiphene, and (Z)-clomiphene was detectable in feces after 1 month of medication (14,18).
Based on the hypothetical theory, the concentrations of active metabolites were predicted to be lower in IM or PM group; however, CC metabolite levels showed considerable variations within IM and EM group. There were not much of differences in concentrations of metabolites between EM and IM group. The relationships between the CYP2D6 genotypes and concentrations of metabolites as well as drug response rate were not clear in this study and could be due to following reasons. We divided the patients into the CC responder and non-responder group based on one cycle of treatment with relatively low CC loading dose. It may influence the degree of effects. Other unknown factors could have influenced the result, as there are many different clinical settings and pathogenesis of infertility.
Our study has several limitations. PMs were not included in this study because less than 1% of Korean has PM phenotype (10,11). Non-standardized collection time was also the limitations of this study. We tried to set the collection day on day 10, but we could not restrict the exact collection day since this was an observational study. The visiting day is decided based on the individual's expected day of ovulation response, and actual collection time was between 8 to 13 days. Lastly, making a clinical decision on drug response after just one cycle of CC treatment was also the limitation.
In conclusion, CC non-responders showed significantly lower (E)-clomiphene concentration than responders, and also showed a trend of decreased concentrations of active metabolites. However, no significant differences in metabolite concentrations were observed between EM and IM phenotype patients. We confirmed that the CC and the metabolite concentrations were different according to the ovulation status. However, our results do not provide evidence for the contribution of CYP2D6 polymorphism to either drug response or CC concentrations. More future studies involving infertility patients may be needed to clarify whether CYP2D6 genotype or phenotype can predict the degree of response to CC.
ACKNOWLEDGMENT
We would like to thank Thomas E. Mürdter and Matthias Schwab (Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany) for quantification of clomiphene and its metabolites by LC-MS/MS from the samples.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study concept and design: Chun S, Ji M. Data acquisition: Ji M, Kim KR. Data analysis: Lee W, Ji M. Writing of the manuscript: Ji M, Choe WH. Editing the manuscript: Chun S, Choe WH. Interpretation of the data and review of the draft: Chun S, Min WK, Kim KR, Lee W.
References
- 1.Vause TD, Cheung AP, Sierra S, Claman P, Graham J, Guillemin JA, Lapensée L, Steward S, Wong BC Society of Obstetricians and Gynaecologists of Canada. Ovulation induction in polycystic ovary syndrome: No. 242, May 2010. Int J Gynaecol Obstet. 2010;111:95–100. doi: 10.1016/j.ijgo.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Koo YH, Na YJ. The infertility treatment related with polycystic ovary syndrome. Korean J Obstet Gynecol. 2010;53:869–880. [Google Scholar]
- 3.Nasseri S, Ledger WL. Clomiphene citrate in the twenty-first century. Hum Fertil (Camb) 2001;4:145–151. doi: 10.1080/1464727012000199212. [DOI] [PubMed] [Google Scholar]
- 4.Mürdter TE, Kerb R, Turpeinen M, Schroth W, Ganchev B, Böhmer GM, Igel S, Schaeffeler E, Zanger U, Brauch H, et al. Genetic polymorphism of cytochrome P450 2D6 determines oestrogen receptor activity of the major infertility drug clomiphene via its active metabolites. Hum Mol Genet. 2012;21:1145–1154. doi: 10.1093/hmg/ddr543. [DOI] [PubMed] [Google Scholar]
- 5.Ganchev B, Heinkele G, Kerb R, Schwab M, Mürdter TE. Quantification of clomiphene metabolite isomers in human plasma by rapid-resolution liquid chromatography-electrospray ionization-tandem mass spectrometry. Anal Bioanal Chem. 2011;400:3429–3441. doi: 10.1007/s00216-011-5045-9. [DOI] [PubMed] [Google Scholar]
- 6.Ghobadi C, Gregory A, Crewe HK, Rostami-Hodjegan A, Lennard MS. CYP2D6 is primarily responsible for the metabolism of clomiphene. Drug Metab Pharmacokinet. 2008;23:101–105. doi: 10.2133/dmpk.23.101. [DOI] [PubMed] [Google Scholar]
- 7.Homburg R. Clomiphene citrate--end of an era? A mini-review. Hum Reprod. 2005;20:2043–2051. doi: 10.1093/humrep/dei042. [DOI] [PubMed] [Google Scholar]
- 8.Hersberger M, Marti-Jaun J, Rentsch K, Hänseler E. Rapid detection of the CYP2D6*3, CYP2D6*4, and CYP2D6*6 alleles by tetra-primer PCR and of the CYP2D6*5 allele by multiplex long PCR. Clin Chem. 2000;46:1072–1077. [PubMed] [Google Scholar]
- 9.Jang YH, Lim SB, Kim MJ, Chung HJ, Yoo HW, Byeon JS, Myung SJ, Lee W, Chun S, Min WK. Three novel mutations of the APC gene in Korean patients with familial adenomatous polyposis. Cancer Genet Cytogenet. 2010;200:34–39. doi: 10.1016/j.cancergencyto.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Lee SY, Sohn KM, Ryu JY, Yoon YR, Shin JG, Kim JW. Sequence-based CYP2D6 genotyping in the Korean population. Ther Drug Monit. 2006;28:382–387. doi: 10.1097/01.ftd.0000211823.80854.db. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Lee SS, Jung HJ, Kim HS, Park SJ, Yeo CW, Shin JG. Discovery of novel functional variants and extensive evaluation of CYP2D6 genetic polymorphisms in Koreans. Drug Metab Dispos. 2009;37:1464–1470. doi: 10.1124/dmd.108.022368. [DOI] [PubMed] [Google Scholar]
- 12.Zanger UM, Raimundo S, Eichelbaum M. Cytochrome P450 2D6: overview and update on pharmacology, genetics, biochemistry. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:23–37. doi: 10.1007/s00210-003-0832-2. [DOI] [PubMed] [Google Scholar]
- 13.Meyer UA. Pharmacogenetics - five decades of therapeutic lessons from genetic diversity. Nat Rev Genet. 2004;5:669–676. doi: 10.1038/nrg1428. [DOI] [PubMed] [Google Scholar]
- 14.Ghobadi C, Mirhosseini N, Shiran MR, Moghadamnia A, Lennard MS, Ledger WL, Rostami-Hodjegan A. Single-dose pharmacokinetic study of clomiphene citrate isomers in anovular patients with polycystic ovary disease. J Clin Pharmacol. 2009;49:147–154. doi: 10.1177/0091270008328096. [DOI] [PubMed] [Google Scholar]
- 15.Mahran A, Abdelmeged A, El-Adawy AR, Eissa MK, Shaw RW, Amer SA. The predictive value of circulating anti-Müllerian hormone in women with polycystic ovarian syndrome receiving clomiphene citrate: a prospective observational study. J Clin Endocrinol Metab. 2013;98:4170–4175. doi: 10.1210/jc.2013-2193. [DOI] [PubMed] [Google Scholar]
- 16.Kim JY, Yi G, Kim YR, Chung JY, Ahn JH, Uhm YK, Jee BC, Suh CS, Kim SH. Association between serum anti-Müllerian hormone level and ovarian response to mild stimulation in normoovulatory women and anovulatory women with polycystic ovary syndrome. Clin Exp Reprod Med. 2013;40:95–99. doi: 10.5653/cerm.2013.40.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurabayashi T, Suzuki M, Fujita K, Murakawa H, Hasegawa I, Tanaka K. Prognostic factors for ovulatory response with clomiphene citrate in polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2006;126:201–205. doi: 10.1016/j.ejogrb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Dickey RP, Holtkamp DE. Development, pharmacology and clinical experience with clomiphene citrate. Hum Reprod Update. 1996;2:483–506. doi: 10.1093/humupd/2.6.483. [DOI] [PubMed] [Google Scholar]


