Abstract
Rationale
Novelty and sensation seeking (NSS) predisposes humans and rats to experiment with psychostimulants. In animal models, different tests of NSS predict different phases of drug dependence. Ultrasonic vocalizations (USV) are evoked by psychomotor stimulants and measure the affective/motivation response to stimuli, yet the role NSS has on USV in response to amphetamine is not determined.
Objectives
Determine if individual differences in NSS and USV can predict locomotor and USV response to amphetamine (0.3, and 1.0 mg/kg) at different exposure lengths.
Methods
Thirty male rats were tested for their response to novelty (IEN), choice to engage in novelty (NPP), and heterospecific play (H-USV). Rats were administered non-contingent amphetamine or saline for 7 exposures and USV and locomotor activity were measured. After a 14-day rest, rats were administered a challenge dose of amphetamine.
Results
Regression analyses indicated that amphetamine dose-dependently increased locomotor activity and the NPP test negatively predicted treatment-induced locomotor activity. The H-USV test predicted treatment-induced frequency modulated (FM) USV, but the strength of prediction depended on IEN response.
Conclusions
Results provide evidence that locomotor activity and FM USV induced by amphetamine represent different behavioral responses. The prediction of amphetamine-induced FM USV by the H-USV screen was changed by the novelty response, indicating that the affective value of amphetamine—measured by FM USV—depends on novelty response. This provides evidence that higher novelty responders may develop a tolerance faster and may escalate intake faster.
Keywords: Amphetamine, Novelty/Sensation Seeking, Ultrasonic Vocalization, Individual Differences, Prediction, Sensitization, Locomotor Activity
Novelty and sensation seeking (NSS) is a trait in humans that predisposes individuals to experiment with drugs of abuse (Zuckerman. 1986). Rodent models of NSS have been developed to understand how NSS contributes to increased addiction vulnerability. The inescapable novelty test (IEN) challenges an animal with a novel-unavoidable open field and the resulting locomotor activity is measured. Higher locomotor activity is interpreted as a greater response to novelty (Piazza et al. 1989; Piazza et al. 1990). High novelty responders in the IEN test show a greater locomotor response to acute low unit doses of psychomotor stimulants when compared to their low responder counterparts (Hooks et al. 1991; Hooks et al. 1992; Mathews et al. 2010). High responders also show greater behavioral sensitization to psychostimulants than low responders after repeated administrations (Hooks et al. 1992; Piazza and Deroche Gamonet. 2013).
To more fully characterize the relationship between novelty and drug use additional NSS tests have been examined. The novelty place preference (NPP) test provides rats the choice to engage a novel environment. High novelty preferring rats show a propensity to develop addiction-like criteria and transition to compulsive cocaine use, while low novelty preferring rats do not demonstrate similar behavior (Belin et al. 2011). Interestingly, high responding rats in the IEN test do not demonstrate addiction-like criteria and numerous experiments have determined that the behavioral responses observed in the IEN and NPP tests are not correlated (Bardo et al. 1996; Cain et al. 2005; Cain et al. 2009; Belin et al. 2011; Bardo et al. 2013) or show a negative correlation (Cain et al. 2004; Beckmann et al. 2011), suggesting that these tests are measuring different traits or that novelty in rodents is multidimensional.
Given the lack of correlation between the tests (Bardo et al. 1996; Cain et al. 2005; Belin et al. 2011; Bardo et al. 2013) and the differences in predicting different phases of the drug response (Bardo et al. 2013; Piazza and Deroche Gamonet. 2013), the current experiment will determine if individual differences in NSS are related to ultrasonic vocalizations (USV). USV show great inter-individual variability, while maintaining intra-individual stability (Taracha et al. 2012), suggesting they can be used as a tool to understand drug response. Research examining USV and drugs of abuse has primarily focused on cocaine and amphetamine (Burgdorf et al. 2001; Ahrens et al. 2009; Ma et al. 2010; Barker et al. 2010; Wright et al. 2010; Brudzynski et al. 2011a; Brudzynski et al. 2011b; Maier et al. 2012; Ahrens et al. 2013), but other research has examined caffeine, ethanol, MDMA, morphine, and nicotine (Covington and Miczek. 2003; Simola et al. 2010; Wright et al. 2012; Simola et al. 2012; Sadananda et al. 2012).
Twenty-two kHz vocalizations are indicative of a negative affective state, because they are evoked by aversive stimuli including predatory presentation (Blanchard et al. 1991), drug withdrawal (Mutschler and Miczek. 1998; Covington and Miczek. 2003), tail shock (van der Poel, A M et al. 1989), and suboptimal drug reinforcement (Barker et al. 2014) after established dependence. Conversely, 50 kHz USV are indicative of positive affective state (Knutson et al. 2002) and are induced upon receipt of conditioned and unconditioned rewards, including: rewarding brain stimulation (Burgdorf et al. 2000; Burgdorf et al. 2007), psychostimulants (see above), and juvenile play with another rat or through experimenter-administered tickling or heterospecific play (Knutson et al. 1998; Burgdorf and Panksepp. 2001; Burgdorf et al. 2005). Researchers using the experimenter tickling procedure or heterspecific test (H-USV), tickle rats with fast-finger movements in 15s intervals for 2 minutes. Tickling reliably evokes 50 kHz USV and is a way to test rats for USV response prior to drug administrations (Brudzynski et al. 2011b).
Fifty kHz USV also reflect dose dependent differences in cocaine self-administration (Barker et al. 2010) and are specifically sensitive to differences in optimal cocaine satiation (Barker et al. 2014) and sucrose satiation (Coffey et al. 2013), suggesting USV are an indicator of subjective reinforcer magnitude and affective motivation. The magnitude of conditioned place preference induced by cocaine and amphetamine is positively correlated with 50 kHz USV (Burgdorf et al. 2007; Meyer et al. 2012; Ahrens et al. 2013), providing strong evidence that the incentive value of a reinforcer can be measured with USV and that the incentive value may be related to the initial affective assessment of a reinforcer.
Research suggests that breeding for high USV calling does alter locomotor response to amphetamine, (Brudzynski et al. 2011a), but other research has generally determined that locomotor sensitization and affective sensitization are separate (Taracha et al. 2012; Ahrens et al. 2013; Taracha et al. 2014). However, traditional locomotor sensitization paradigms usually administer 5–7 systemic drug injections (Kalivas and Stewart. 1991; Hooks et al. 1991; Arndt et al. 2014), while USV sensitization paradigms follow a two injection protocol (TIPS) spaced 7 days apart (Taracha et al. 2014). Importantly, when amphetamine is administered systemically USV sensitization only occurred in high vocalizer rats. In another article, individual differences in USV sensitization were not observed in the TIPS protocol and were only observed only after daily repeated injections (Taracha et al. 2012).
Taken together, the research suggests that individual differences in NSS and USV are related to the propensity for psychostimulant addiction. The current study aimed to determine if individual differences in NSS or affective response induced by the H-USV test (tickling) could collectively or uniquely predict locomotor and USV response to amphetamine in a prolonged behavioral sensitization paradigm. In addition, many NSS and USV experiments typically use median splits or similar techniques to create artificial groups which can create Type I or II error (Irwin et al. 2003; Cain et al. 2005; De Coster et al. 2011). Therefore, hierarchical regression analyses were used to maintain the continuous scaling of the NSS and USV responses to understand if these tests could collectively or uniquely predict locomotor and USV response to acute and chronic amphetamine or saline.
Methods
Animals
Thirty adolescent male Sprague-Dawley rats were purchased from Charles River Laboratories. Rats were 30 days old on arrival and were individually housed in a temperature and humidity controlled colony room. Rat cages were housed in standard transparent polyurethane cages with Carefresh® bedding with food and water available ad libitum for the duration of the experiment. After arrival rats were handled daily with experimentation beginning 10 days after arrival. Rats were maintained on a 12:12 light:dark cycle with lights on from 7:00–19:00. All experimentation occurred in the light cycle. The procedures were approved by the Kansas State University Institutional Animal Care and Use Committee and the Guide for the care and Use of Animals (National Research Council, 2011).
Drugs
d-amphetamine sulfate (Sigma, St. Louis, MO) was dissolved in 0.9% saline and was administered at 0.3 & 1.0 mg/kg doses (i.p.) in a 1 ml/kg volume. The doses tested were chosen to produce locomotor activity without producing stereotyped behaviors (Pijnenburg et al. 1975) and to allow for observation of individual differences in response to novelty because these differences are most robust at low unit doses (Hooks et al. 1992; Cain et al. 2005).
Apparatus
The inescapable novelty test (IEN) was conducted in activity chambers measuring 46.6 × 46.6 × 46.6 cm. Each chamber was constructed of transparent plexiglass walls and plastic flooring covered with pine shavings. A 16 × 16 photocell array surrounded the locomotor chamber and was 2.54 cm above the plastic flooring. Each photocell was spaced 2.54 cm apart (Coulbourn Instruments, TruScan 2.01) and measured the amount of horizontal movement in centimeters. The horizontal movement was recorded in 5-minute blocks of time for each session and summed to yield a total distance traveled. A white noise generator (70 dB) was used to create background noise to mask external sounds.
The novelty place preference test (NPP) was conducted in a three compartment preference chamber constructed of Plexiglas. Each end compartment measured 29 × 23 × 45 (L×W×H) cm. The walls of one compartment were white and the floor was made of wire mesh (13 × 13 mm). Pine shavings were placed in the litter tray located beneath the wire mesh floor. The walls of the other end compartment were black and the floor was made of 15 metal rods (6 mm in diameter) spaced 2 cm apart center to center. Compressed pellet bedding was placed in the litter tray located beneath the metal rod floor. The smaller center compartment was 19 × 23 × 45 (L×W×H) cm. Its walls and floor were painted grey and constructed entirely of Plexiglas. The walls that separated the three compartments were replaced on the test day with similarly painted walls that had a 10.5×10.5 cm opening at the bottom center to allow access to all compartments.
The heterospecific test (H-USV) and all ultrasonic recording were conducted in a separate room, and each animal was tested individually. During the heterospecific test, the recording chamber was comprised of a standard transparent shoebox cage. The cage floor was covered with Carefresh® litter bedding. During amphetamine training sessions, the recording chamber was comprised of a standard opaque shoebox cage. In addition to the cage color difference, the cage floor was covered with compressed pellet bedding to provide an additional contextual cue.
Ultrasonic equipment and analysis
USV were recorded using an ultrasonic microphone (Ultramic 200K) and recording software SEAwave (Sound Emission Analyzer). USV of all call types and frequencies were counted automatically and scored similar to Ahrens et al. (2009) and Burgdorf et al. (2007). The Ultramic 200K and recording software interfaced with a separate computer that automatically saved the sound file for subsequent analysis. AviSoft SASLab Pro Bioacoustics sound analysis software was used to generate a spectrogram, and score the parameters of each USV ranging from 18–100 kHz and duration of 10 ms. A trained researcher blind to experimental conditions scored the shape of the USV as flat, or frequency modulated (FM) (Garcia et al. 2015). During shape analysis all USV were verified using the playback function of SASLab. Operational definitions are as follows: Flat USV had no visual modulations. Simple FM USV had 1 or 2 modulations, change in frequency that did not have trill or step-trill components. FM USV were operationally defined as having 3 or more modulations and included trill USV, and step-like USV that had trills at the beginning or end of the call. Modulations were operationally defined as having a greater than ~3kHz change in frequency. The parameter values were copied into Microsoft Excel and categorized into 22 kHz (20–28 kHz) and 50 kHz (35–100 kHz) based on peak frequency. Calls in the range of 28,001–34,999 were not classified and not included in statistical analysis. The number of unclassified was ~3–5% depending on the animal. The present study examined over 30,000 USV calls. The H-USV screen is designed to probe for 50 kHz USV, and as a result, minimal 22 kHz USV were observed. Simple FM USV (1 or 2 modulations) did not predict amphetamine-induced USV. Therefore, the analyses for 22 kHz and simple FM USV are not presented or discussed further.
Procedure
All rats (N= 30) received all tests in the same order: IEN, NPP, H-USV. The order of tests was determined by prior research that used regression statistical models (Cain et al. 2005). The first day the rats were tested for their response to novelty with the IEN test. Rats were placed inside of the chamber for 30 minutes, and total distance traveled (cm) was recorded by the computer. The next day rats were tested for their preference for novelty with the NPP test. Rats were individually placed and restricted to either the white or black end compartments of the place preference chamber for 30 minutes for two consecutive days. On the test day, the rats were placed in the neutral grey center and allowed access to habituated and novel side for 15 minutes. Time spent on each the habituated and novel side was recorded and a preference ratio was calculated [time novel / (total time - minus the grey)]. A rat was considered to be in a compartment when both front paws were in a compartment. The rats were tested for their affective response to rewarding stimuli using the H-USV test. Rats were individually transported to a separate room and placed inside an arena where ultrasonic recordings occurred. Rats were then ‘tickled’ for 15s followed by 15s of no stimulation. This cycle repeated for two minutes for three consecutive days. On the 4th day, the procedure was identical, but the microphone recorded USV of all call types.
After all tests were completed, rats were block randomized to either the saline or amphetamine (0.3 or 1.0 mg/kg) conditions, such that the higher and lower NSS rats and USV callers were equally distributed across all treatment groups. Rats were transported to a holding room adjacent to the USV recording room. Rats were injected (intraperitoneally i.p) with their respective treatment and placed back in their homecage for 15 minutes. After 15 minutes, the injected rat was transported to the recording room and placed in the arena with a cage lid. The number and shape of USV were recorded for two minutes (15–17 min. post injection). After USV recording was finished, the rat was transported to the activity chambers and total distance traveled (cm) was recorded for 60 min. This procedure was completed for a total of 7 treatments over 14 days. Ultrasonic recording occurred on Days 1, 7, and Challenge day. Days 2–6 the microphone was on, but did not record USV. This was intentionally done because of the laborious nature of scoring USV. After the 7th treatment all rats went on a 14-day break and were not administered any drug, and handling was limited to bedding/cage changes. Then, all rats were given a challenge dose of amphetamine (0.3 or 1.0 mg/kg) to test for behavioral and affective sensitization. Rats previously administered saline were divided equally across the amphetamine treatments, such that half received the 0.3 mg/kg and half received 1.0 mg/kg. USV and locomotor activity were recorded as previously described.
Analyses
To facilitate comparison with previous literature, we calculated and presented the mean ± SEM locomotor and USV responses for each test session and conducted ANOVAs to determine if treatment-induced differences existed regardless of individual differences. To examine the relationship between NSS, H-USV, and the response to amphetamine, we used hierarchical regression because each of the independent variables (IEN, NPP, and H-USV) are continuous. When independent variables are continuous the appropriate analysis is a regression, because it does not require median splitting that is required to accommodate ANOVA. Median splitting can result in the introduction of Type I or Type II error (Maxwell et al. 1993; Irwin et al. 2003; De Coster et al. 2011). Regression allows for multiple continuous or categorical variables to be entered together to predict a dependent variable. Therefore, total distance traveled (IEN), place preference ratio (NPP) and FM USV counts (H-USV) were used to predict locomotor activity and USV after their respective treatments. All FM USV presented in results were the 50 kHz variety. Two separate regressions were conducted for each test session. The first determined if the IEN, NPP, and H-USV tests predicted the locomotor response to amphetamine or saline. The second determined if the IEN, NPP, and H-USV tests predicted amphetamine or saline-induced FM USV calling. All hierarchical models entered the IEN, NPP, and H-USV tests in the first step. For the second step, saline was selected to be the comparison group, and the low and high doses were tested against the saline group to determine if they could add more prediction to the overall model. Finally, all two-way interactions were tested independently and if significant, were probed using simple slope analyses. The simple slope determines if the slope is significantly different from zero. Meaningful models were assessed with R2, which represents the total variance accounted for in the dependence variable by the predictor variables. Significant R2 change was determined by F statistic and p<.05. β coefficients are an estimate of effect size in standard deviations (Nakagawa and Cuthill. 2007; Nathans. 2012). Significant β coefficients above .40 (in absolute value) are considered strong effects, and generally statistically significant at p<.05. Positive or negative values simply describe the direction of the effect. All significance was set at p ≤ .05. The bivariate correlations between IEN, NPP, and number of USV in response to H-USV are described in another manuscript (Garcia et al. 2015).
Results
Locomotor and affective response to acute amphetamine: Session 1
Predicting Total Distance Traveled (cm)
The overall model accounted for a significant amount of variance in total distance traveled in response to the first treatment (R2 = .28, p < .05). There was a main effect of NPP, such that higher novelty preferring rats showed less distance traveled on session 1, demonstrating a significant negative relationship (β = −.48, p < .01; see Fig. 1). There was no effect of IEN and H-USV, meaning they did not predict the total distance traveled to treatment on the first session. Entry of treatment condition in the 2nd step accounted for significantly more variance (R2 = .76, p < .001). There was a main effect of drug, such that the low and high doses increased distance traveled. The drug effects were positive relationships (β = .51, p < .001; β = .89, p < .001), indicating that rats in the low dose and high dose groups traveled .51 and .89 standard deviations more than the saline treated rats. All possible 2-way interactions were tested independently by entry into the model and then the interaction term was removed for a subsequent interaction tests. When predicting total distance traveled on session 1 none of the interactions significantly increased the model’s prediction of the dependent variable (Table 1).
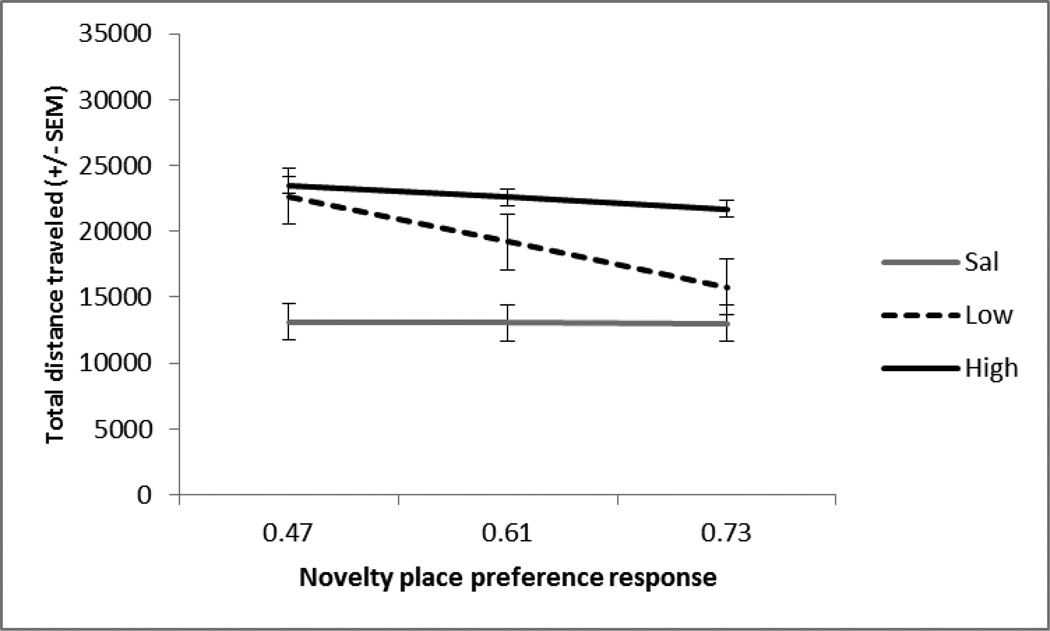
Fig. 1.
Session 1. The mean +/− SEM total distance traveled (cm) in response to saline (n=10), low (0.3 mg/kg; n=10) or high (1.0 mg/kg; n=10) dose of amphetamine at the mean and 1SDabove and below the mean of the NPP response (x-axis). Both amphetamine doses increased locomotor activity compared to saline-treated rats, p<.001, and the NPP test had a significant negative relationship with locomotor activity, p<.01. The slopes are not significantly different from one another.
Table 1.
Hierarchical regression analysis predicting total distance traveled in response to the first day of treatment. Interactions were entered independently, such that interactions were tested then removed from the model. R2 describes the total variance in the dependent variable accounted for by the independent variables in each step. β is a standardized measure of effect size in standard deviation units. β’s greater than .40 (absolute value) are considered strong effects are typically statistically significant. The t test, tests if the β is significantly different from zero.
| Step and predictor variable | R2 | β | t | |
|---|---|---|---|---|
| Step 1 | .28* | |||
| IEN | .18 | 1.05 | ||
| NPP | −.48 | 2.86** | ||
| H-USV FM | .09 | 0.55 | ||
| Step 2 | .76*** | |||
| Low 0.3 mg/kg | .51 | 4.25*** | ||
| High 1.0 mg/kg | .89 | 6.90*** | ||
| Step 3 | ||||
| Low 0.3 mg/kg × IEN | .79 | .20 | 1.27 | |
| High 1.0 mg/kg × IEN | .22 | 1.75 | ||
| Low 0.3 mg/kg × NPP | .77 | −.18 | 1.34 | |
| High 1.0 mg/kg × NPP | −.15 | 0.57 | ||
| Low 0.3 mg/kg × H-USV FM | .77 | .10 | 0.56 | |
| High 1.0 mg/kg × H-USV FM | −.02 | 0.11 | ||
| IEN × NPP | .77 | −.14 | 1.08 | |
| IEN × H-USV FM | .77 | .09 | 0.89 | |
| NPP × H-USV FM | .76 | −.08 | 0.66 |
p < .05;
p < .01;
p < .001
Predicting FM USV
The next regression model accounted for a significant amount of variance in FM USV in response to the first treatment, (R2 = .56, p < .001). There was no effect of IEN and NPP tests as evidence by no predictive relationship with FM USV in response to acute amphetamine or saline treatment. There was a main effect of the H-USV test, indicating higher FM USV callers in the H-USV screen vocalized more during session 1. This effect of H-USV test was positive, (β = .67, p < .001). In the 2nd step, the high and low dose of amphetamine were entered to determine if they changed FM USV called compared to saline. There was no main effect of drug, indicating the low or high dose did not change the number of FM USV when compared to saline, (β = −.02, p >.05; β = .04, p > .05). Importantly, tests of interactions in the third step revealed marginally significant interactions between drug and H-USV test (R2 = .66, p = .06). Specifically, the low amphetamine dose interacted with the H-USV test, such that higher callers during the H-USV test vocalized less when given the low dose of amphetamine, and FM USV calling was not affected by the saline or high dose of amphetamine (figure not shown). Figure 2 shows a non-significant interaction of the IEN × H-USV tests to depict how the relationship changed across repeated injections. All other 2-way interactions were entered into the model but were not significant (Table 2).
Fig. 2.
Session 1. The mean +/− SEM total number of frequency modulated 50 kHz USV at low (−1SD;), mean, and high (+1SD) levels of inescapable novelty response at the mean and 1SD above and below the mean of the H-USV test response (x-axis). The H-USV response had a significant positive relationship with FM USV, p<.001 (n=30). The slopes of the lines are not significantly different from one another.
Table 2.
Hierarchical regression analysis predicting FM 50 kHz USV in response to the first day of treatment. Interactions were entered independently, such that interactions were tested then removed from the model. See Table 1’s description for the interpretation of R2, β, and t.
| Step and predictor variable | R2 | β | t | |
|---|---|---|---|---|
| Step 1 | .56*** | |||
| IEN | −.24 | 1.82 | ||
| NPP | −.18 | 1.35 | ||
| H-USV FM | .67 | 5.12*** | ||
| Step 2 | .56 | |||
| Low 0.3 mg/kg | −.02 | 0.12 | ||
| High 1.0 mg/kg | .04 | 0.23 | ||
| Step 3 | ||||
| Low 0.3 mg/kg × IEN | .65 | .01 | 0.03 | |
| High 1.0 mg/kg × IEN | −.34 | 2.05^ | ||
| Low 0.3 mg/kg × NPP | .63 | −.25 | 1.44 | |
| High 1.0 mg/kg × NPP | .14 | 0.42 | ||
| Low 0.3 mg/kg × H-USV FM | .66 | −.48 | 2.09* | |
| High 1.0 mg/kg × H-USV FM | −.08 | 0.37 | ||
| IEN × NPP | .56 | .07 | 0.37 | |
| IEN × H-USV FM | .59 | −.19 | 1.37 | |
| NPP × H-USV FM | .57 | −.10 | 0.59 |
p = .053;
p < .05;
p < .01;
p < .001
Locomotor and affective response to repeated amphetamine: Session 7
Mean locomotor activity (cm)
Analysis of the mean distance traveled (cm) during each locomotor session revealed that amphetamine-induced locomotor activity increased with repeated amphetamine injections with the highest amphetamine dose resulting in greatest distance traveled (Fig 3).
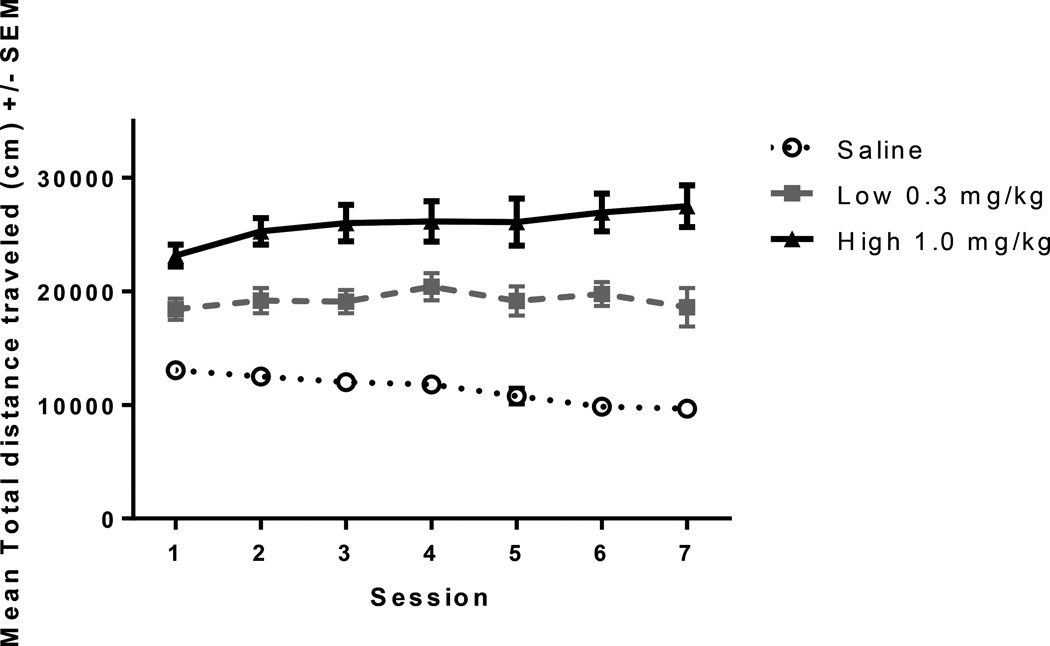
Fig. 3.
Session locomotor data. Mean +/− SEM total distance traveled in response to saline (n=10), low (0.3 mg/kg amphetamine (n=10) or high (1.0 mg/kg amphetamine (n=10) by session number (x-axis). ANOVA indicated all treatments affected total distance traveled differently, F(2, 27)=46.41, p<.001. Significant differences between all doses across all sessions, p<.01 Paired t-test comparing session 1 to session 7 revealed a decrease in saline-treated, no change in low dose, and an increase in high dose treated rats, p’s<.05.
Predicting Total Distance Traveled (cm)
The IEN, NPP or H-USV tests did not account for a significant amount of variance, (R2 = .09, p > .05). Entry of the drug treatment did account for a significant amount of variance, (R2 = .74, p < .001), indicating a main effect of the low and high doses of amphetamine. Each dose significantly increased locomotor activity compared to the saline treated rats (β = .51, p < .001; β = 1.04, p < .001). All 2-way interactions were tested and none resulted in a significant amount of additional variance accounted for when added to the model. The low dose differentially affected high preferring and low preferring rats but this was not significant (Fig 4; Table 3).
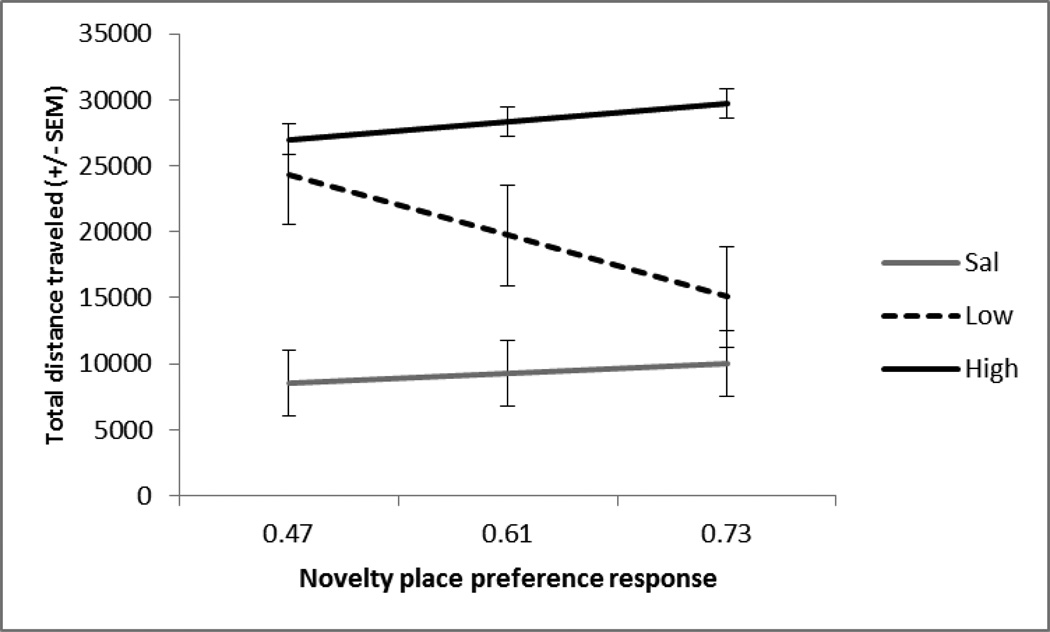
Fig. 4.
Session 7. The mean +/− SEM total distance traveled (cm) in response to saline (n=10), low (0.3 mg/kg; n=10) or high (1.0 mg/kg; n=10) dose of amphetamine at the mean and 1SD above and below the mean of the NPP response (x-axis). Repeated amphetamine at the low and high dose significantly increased locomotor activity compared to saline, p<.001. The slopes of the lines are not significantly different from one another, suggesting only a dose-dependent effect.
Table 3.
Hierarchical regression analysis predicting total distance traveled in response to the seventh day of treatment. Interactions were entered independently, such that interactions were tested then removed from the model. See Table 1’s description for the interpretation of R2, β, and t.
| Step and predictor variable | R2 | β | t | |
|---|---|---|---|---|
| Step 1 | .09 | |||
| IEN | .03 | 0.15 | ||
| NPP | −.26 | 1.36 | ||
| H-USV FM | .14 | 0.72 | ||
| Step 2 | .74*** | |||
| Low 0.3 mg/kg | .51 | 4.11*** | ||
| High 1.0 mg/kg | 1.04 | 7.77*** | ||
| Step 3 | ||||
| Low 0.3 mg/kg × IEN | .77 | −.03 | 0.18 | |
| High 1.0 mg/kg × IEN | .19 | 1.39 | ||
| Low 0.3 mg/kg × NPP | .77 | −.16 | 1.19 | |
| High 1.0 mg/kg × NPP | .06 | 0.22 | ||
| Low 0.3 mg/kg × H-USV FM | .75 | −.01 | 0.04 | |
| High 1.0 mg/kg × H-USV FM | .09 | 0.46 | ||
| IEN × NPP | .75 | −.14 | 1.06 | |
| IEN × H-USV FM | .74 | −.03 | 0.25 | |
| NPP × H-USV FM | .74 | −.01 | 0.09 |
p < .05;
p < .01;
p < .001
Mean number of FM USV
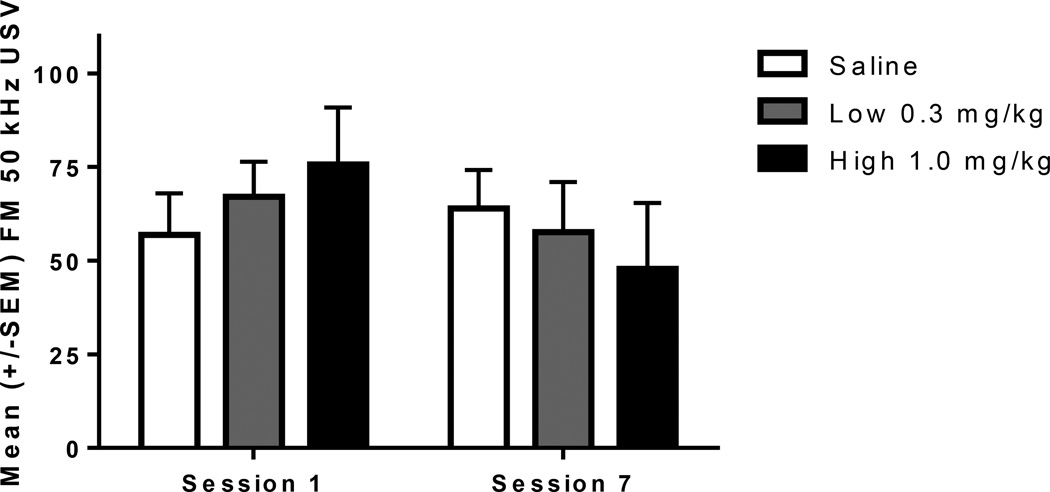
FM USV varied as a function of amphetamine dose and generally decreased with repeated injections (Fig 5).
Fig. 5.
Mean +/− SEM Total FM USV counts for Session 1 and 7 for saline treated (n=10), Low dose 0.3 mg/kg (n=10), and High dose 1.0 mg/kg (n=10). Paired t-test revealed that saline and low dose treated rats did not change FM USV calling, but the high dose decreased FM USV from session 1 to session 7, p<.05.
Predicting FM USV
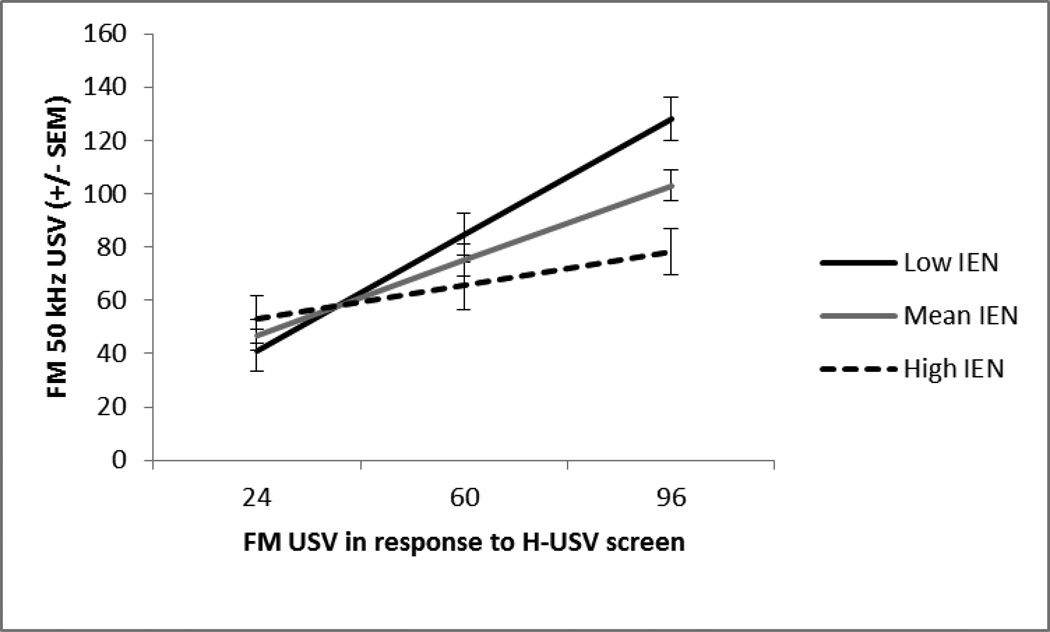
The IEN, NPP, and H-USV tests accounted for a significant amount of variance when predicting FM USV on Session 7 (R2 = .44, p < .005). There was not a main effect of IEN or NPP, meaning the effect was entirely driven by the H-USV. There was a main effect of the H-USV test. The H-USV test positively predicted FM USV(β = .62, p < .001), suggesting the higher FM USV vocalizers in the H-USV test remained the higher vocalizers throughout chronic treatment. In the next step, drug was entered into the regression model and showed that both the low and high dose of amphetamine reduced FM USV when compared to saline, but this was not a significant reduction (β = −.22, p = .22; β = −.35, p = .07). Tests of interactions showed that the IEN and H-USV tests interacted and significantly increased the amount of explained variance in FM USV after 7 exposures (R2 = .63, p = .01). Specifically, the strength of the H-USV’s relationship with treatment-induced FM USV decreases as the IEN response increases (β = −.35, p = .01; Fig 6 and Table 4), but only when the rats received repeated administrations of the high dose. Probing this interaction using simple slope analyses revealed that at high levels of the IEN response the relationship between H-USV test and treatment-induced FM USV remained the same. More importantly, when the IEN response was low, the H-USV test showed a stronger significant positive relationship with amphetamine-induced FM USV (p < .05), but this relationship was dependent on repeated administrations of the high dose of amphetamine. No other 2-way interactions were significant.
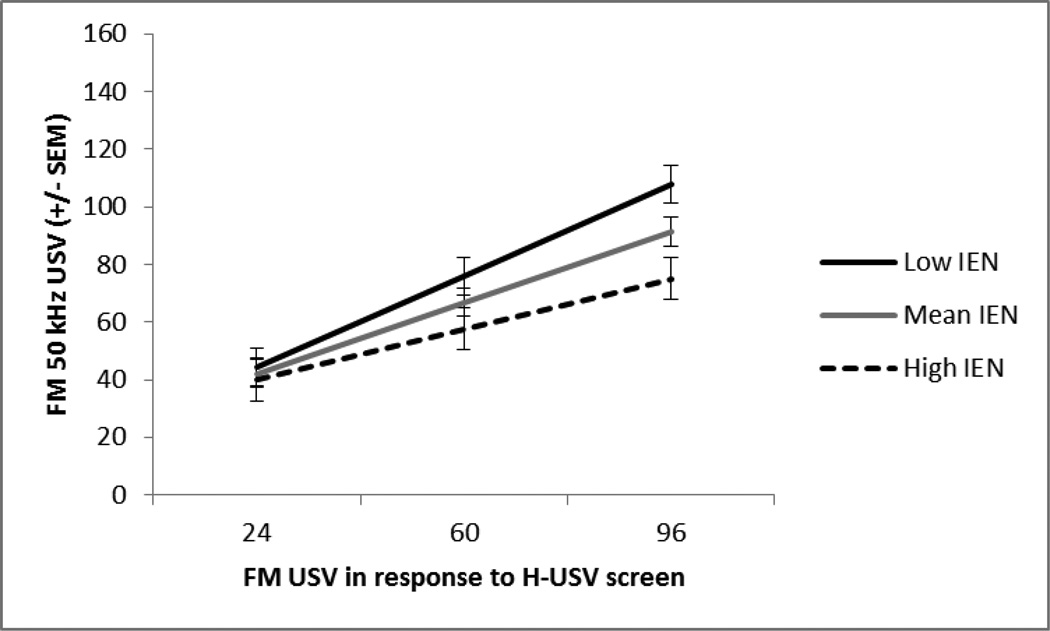
Fig. 6.
Session 7. The mean +/− SEM total number of frequency modulated 50 kHz USV at low (−1SD), mean, and high (+1SD) levels of inescapable novelty response at the mean and 1SD above and below the mean of the H-USV test response (x-axis). The H-USV test’s prediction of treatment-induced FM USV depended on the IEN response. Higher IEN responders vocalized less, p<.01. This interaction was only observed after repeated administrations of the high dose of amphetamine and not the low dose, p<.05.
Table 4.
Hierarchical regression analysis predicting FM 50 kHz USV in response to the seventh day of treatment. Interactions were entered independently, such that interactions were tested then removed from the model. See Table 1’s description for the interpretation of R2, β, and t.
| Step and predictor variable | R2 | β | t | |
|---|---|---|---|---|
| Step 1 | .44** | |||
| IEN | −.24 | 1.61 | ||
| NPP | .02 | 0.16 | ||
| H-USV FM | .62 | 4.22*** | ||
| Step 2 | .52 | |||
| Low 0.3 mg/kg | −.22 | 1.29 | ||
| High 1.0 mg/kg | −.35 | 1.90 | ||
| Step 3 | ||||
| Low 0.3 mg/kg × IEN | .58 | −.10 | 0.46 | |
| High 1.0 mg/kg × IEN | −.31 | 1.72 | ||
| Low 0.3 mg/kg × NPP | .54 | −.18 | 0.94 | |
| High 1.0 mg/kg × NPP | −.35 | 0.93 | ||
| Low 0.3 mg/kg × H-USV FM | .53 | .04 | 0.15 | |
| High 1.0 mg/kg × H-USV FM | .15 | 0.57 | ||
| IEN × NPP | .54 | .18 | 1.02 | |
| IEN × H-USV FM | .63 | −.35 | 2.67** | |
| NPP × H-USV FM | .52 | −.04 | 0.25 |
p < .05;
p < .01;
p < .001
Locomotor and affective response during sensitization test: Challenge Session
Mean locomotor activity (cm)
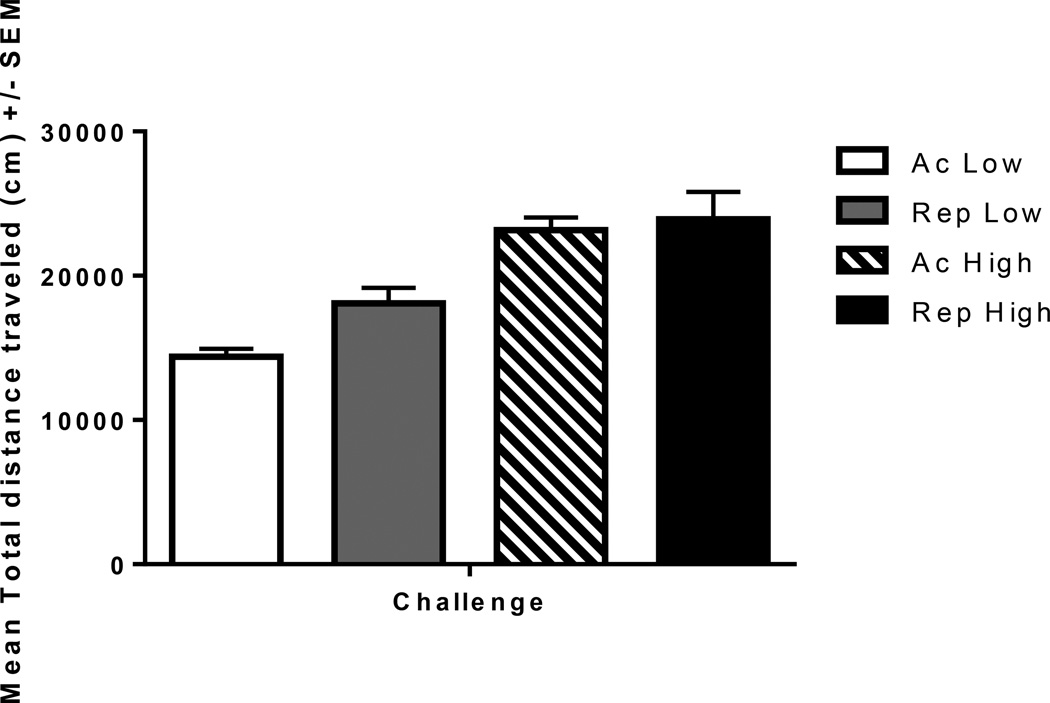
Analysis of the mean distance traveled (cm) during the sensitization test revealed that high dose of amphetamine resulted in more amphetamine-induced locomotor activity than the low dose of amphetamine. However, we did not observe a significant difference between the rats given their first injection of amphetamine versus the rats given their challenge injection (Fig 7).
Fig. 7.
The mean +/− SEM total distance traveled during challenge session. All rats administered amphetamine. Acute Low (Ac Low; n=5), Repeated Low (Rep Low; n=10), Acute High (Ac High; n=5), Repeated High (Rep High; n=10). ANOVA indicated that the high dose increased locomotor activity compared to the low dose, but there were no effects of repeated compared to acute treatment for either dose tested, p>.05.
Predicting Total Distance Traveled (cm)
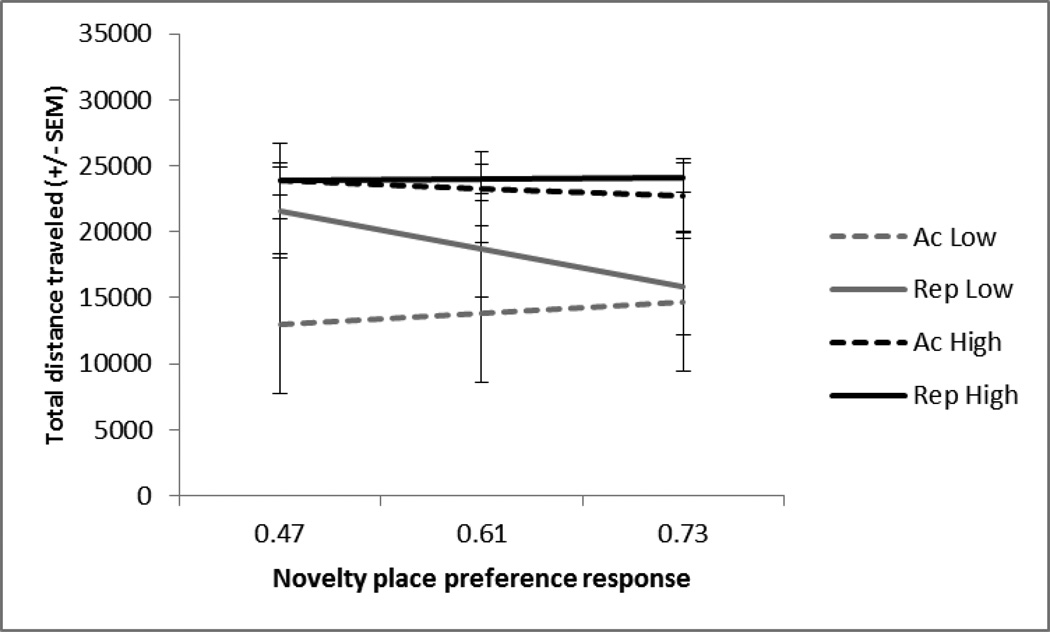
On the challenge session the IEN, NPP, and H-USV tests did not predict amphetamine-induced locomotor activity, (R2 = .10, p > .05). However, entry of the drug in step 2 revealed a main effect of drug as evidenced by the significant amount of explained variance, (R2 = .49, p < .005). There was no main effect of repeated low dose, indicating the repeated low dose did not increase locomotor activity when compared to the rats that were treated with repeated saline and challenged with the low dose of amphetamine, (β = .32, p > .05). Rats previously administered saline but given the high dose displayed greater locomotor activity than the acute low and repeated low dose groups, (β = .62, p < .005). Similarly, the repeated high dose increased locomotor activity when compared to repeated low dose and acute low dose treated rats (β = .83, p < .001). However, rats treated with the repeated high dose did not display greater locomotor activity when compared to the saline-treated rats tested with the high dose for the first time. All 2-way interactions were tested and did not reveal any significant interactions when predicting locomotor response on the challenge session (Fig 8; Table 5).
Fig. 8.
Challenge session. The mean +/− SEM total distance traveled (cm) in response to acute low (Ac-Low; n=5), repeated low (Rep-Low; n=10), acute high (Ac-High; n=5) or repeated high (Rep-High; n=10) doses of amphetamine. Regression lines are plotted at the mean and 1SD above and below the mean of the NPP response (x-axis). The high dose increased distance traveled compared to the low dose but there was not a difference between acute and repeated for either dose of amphetamine tested. None of the tests predicted distance traveled.
Table 5.
Hierarchical regression analysis predicting total distance traveled in response to the challenge day of treatment. Interactions were entered independently, such that interactions were tested then removed from the model. See Table 1’s description for the interpretation of R2, β, and t.
| Step and predictor variable | R2 | β | t | |
|---|---|---|---|---|
| Step 1 | .10 | |||
| IEN | −.08 | 0.44 | ||
| NPP | −.29 | 1.52 | ||
| H-USV FM | .05 | 0.27 | ||
| Step 2 | .49** | |||
| Repeated Low 0.3 mg/kg | .32 | 1.50 | ||
| Acute High 1.0 mg/kg | .62 | 3.17** | ||
| Repeated High 1.0 mg/kg | .83 | 3.56** | ||
| Step 3 | ||||
| Repeated Low 0.3 mg/kg × IEN | .51 | −.11 | 0.29 | |
| Acute High 1.0 mg/kg × IEN | −.12 | 0.42 | ||
| Repeated High 1.0 mg/kg × IEN | .10 | 0.36 | ||
| Repeated Low 0.3 mg/kg × NPP | .50 | −.18 | 0.59 | |
| Acute High 1.0 mg/kg × NPP | −.08 | 0.24 | ||
| Repeated High 1.0 mg/kg × NPP | −.11 | 0.13 | ||
| Repeated Low 0.3 mg/kg × H-USVFM .57 | −.20 | 0.69 | ||
| Acute High 1.0 mg/kg × H-USV FM | −.14 | 0.66 | ||
| Repeated High 1.0 mg/kg × H-USV FM | .20 | 0.71 | ||
| IEN × NPP | .50 | −.19 | 0.92 | |
| IEN × H-USV FM | .48 | .01 | 0.07 | |
| NPP × H-USV FM | .48 | −.05 | 0.24 | |
p < .05;
p < .01;
p < .001
Mean number of FM USV
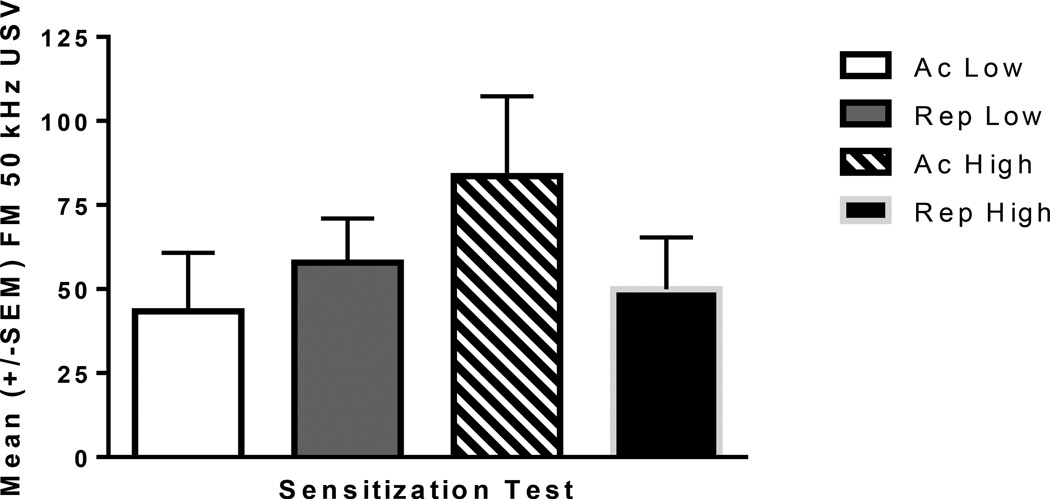
FM USV: The number of FM USV varied as a function of amphetamine dose and history with the rats given their first injection of the high dose of amphetamine vocalizing the most (Fig 9).
Fig. 9.
Mean +/− SEM total FM USV counts for the Challenge session. All rats administered amphetamine: Acute Low (Ac Low; n=5), Repeated Low (Rep Low; n=10), Acute High (Ac High; slash pattern; n=5), Repeated High (Rep High; n=10). There was no dose dependent effect and no effect of repeated treatment.
Predicting FM USV
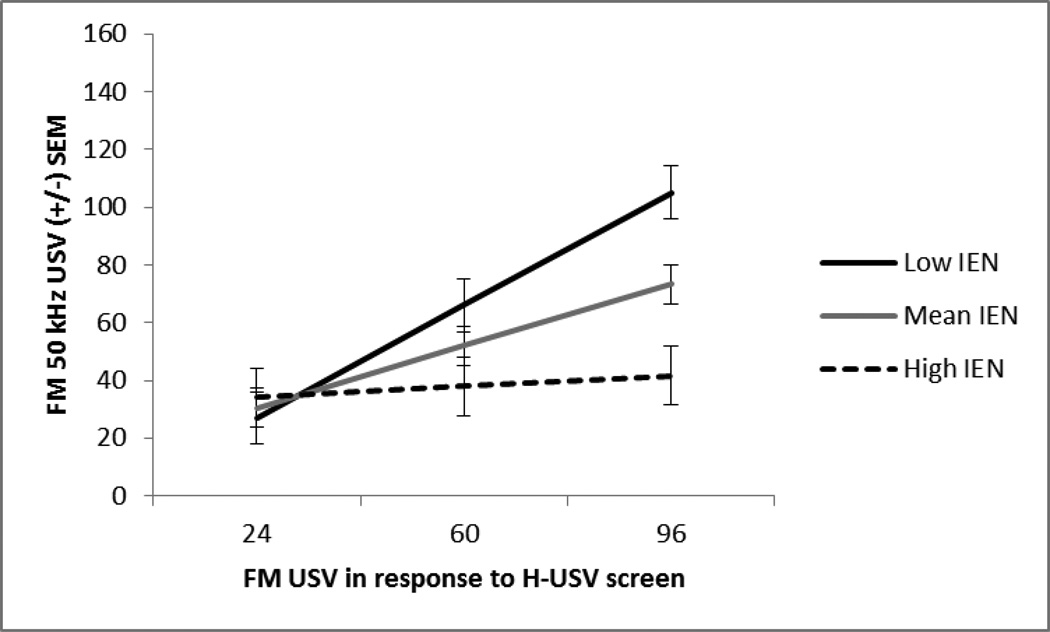
When predicting FM USV on the challenge session, entry of the IEN, NPP, and H-USV tests accounted for a significant amount of explained variance, (R2 = .32, p < .05). There was no effect of NPP test, suggesting no relationship with FM USV. However, the IEN test showed a marginally significant negative relationship, (β = −.32, p = .06), suggesting higher novelty responders emitted less FM USV. There was a main effect of the H-USV test, such that higher vocalizers in the H-USV test remained high throughout the challenge session. The H-USV effect was positive, (β = .45, p < .01). Entry of the drug in the second step revealed no main effect of drug and did not result in more explained variance, (R2 = .46, p > .05), suggesting that the acute low, repeated low, acute high, and repeated high doses did not change FM USV differently (See Table 6). All 2-way interactions were tested. Similar to the interaction on the 7th exposure, the IEN and H-USV tests accounted for more explained variance in FM USV on the challenge session as evidenced by a significant interaction, (R2 = .60, p = .01). The interaction of the IEN and H-USV negatively predicted amphetamine-induced FM USV, (β = −.38, p = .01), suggesting that the relationship between the H-USV test and amphetamine-induced FM USV decreases as novelty response increases. Using simple slopes to probe this interaction revealed similar slope changes observed in session 7. The H-USV test’s relationship with amphetamine-induced FM USV did not change at high levels of the IEN response, but showed a significant positive relationship at low levels of IEN response (Fig 10). No other 2-way interactions were significant.
Table 6.
Hierarchical regression analysis predicting FM 50 kHz USV in response to the challenge of treatment. Interactions were entered independently, such that interactions were tested then removed from the model. See Table 1’s description for the interpretation of R2, β, and t.
| Step and predictor variable | R | β | t | |
|---|---|---|---|---|
| Step 1 | .32* | |||
| IEN | −.32 | 1.98^ | ||
| NPP | −.10 | 0.59 | ||
| H-USV FM | .45 | 2.78** | ||
| Step 2 | .46 | |||
| Repeated Low 0.3 mg/kg | .05 | 0.23 | ||
| Acute High 1.0 mg/kg | .34 | 1.71 | ||
| Repeated High 1.0 mg/kg | −.09 | 0.37 | ||
| Step 3 | ||||
| Repeated Low 0.3 mg/kg × IEN | .53 | −.15 | 0.41 | |
| Acute High 1.0 mg/kg × IEN | .07 | 0.27 | ||
| Repeated High 1.0 mg/kg × IEN | −.31 | 1.18 | ||
| Repeated Low 0.3 mg/kg × NPP | .47 | −.14 | 0.43 | |
| Acute High 1.0 mg/kg × NPP | −.29 | 0.82 | ||
| Repeated High 1.0 mg/kg × NPP | −.67 | 0.45 | ||
| Repeated Low 0.3 mg/kg × H-USVFM .51 | −.17 | 0.55 | ||
| Acute High 1.0 mg/kg × H-USV FM | .17 | 0.76 | ||
| Repeated High 1.0 mg/kg × H-USV FM | .07 | 0.22 | ||
| IEN × NPP | .46 | .06 | 0.30 | |
| IEN × H-USV FM | .59* | −.38 | 2.75** | |
| NPP × H-USV FM | .46 | −.12 | 0.62 | |
p = .059;
p < .05;
p < .01;
p < .001
Fig. 10.
Challenge session. The mean +/− SEM total number of frequency modulated 50 kHz USV at low (−1SD), mean, and high (+1SD) levels of inescapable novelty response at the mean and 1SD above and below the mean of the H-USV test response (x-axis). The H-USV test’s prediction of treatment-induced FM USV depended on the IEN response. Higher IEN responders vocalized less, p<.01.
Discussion
Our data provide strong support that the IEN, NPP, and H-USV tests can uniquely predict the locomotor and affective response to a low and high dose of amphetamine. Furthermore, behavioral sensitization appears to occur across different domains. Locomotor sensitization and affective sensitization, as measured by FM USV, are distinct processes and suggests each can be researched to understand individual differences in amphetamine response.
Generally, when median split statistical techniques are used, high responders in the IEN test show greater locomotor activity in response to low-unit doses of amphetamine, when compared to their low responding counterparts (Hooks et al. 1991). The latter effect was not observed in the current study. This is consistent with previous research using regression analyses to examine the relationship between inescapable novelty and amphetamine self-administration (Cain et al. 2005). However, in the current study the NPP test showed a negative predictive relationship with treatment-induced locomotor activity, such that the highest novelty preferring rats showed the least locomotor activity in response to treatment in Session 1. Interestingly, when chronic amphetamine was administered, the NPP test no longer predicted treatment-induced locomotor activity, suggesting that NPP individual differences are most robust early in drug training and diminish with prolonged drug exposure. The H-USV test did not predict locomotor activity early or late in drug training. This result is consistent with other research suggesting that USV are not related to cocaine or amphetamine-induced locomotor activity (Taracha et al. 2012; Ahrens et al. 2013; Taracha et al. 2014). This result was not observed in the current experiment even when regression was used for statistical analysis and provides strong evidence that locomotor activity and USV in response to psychomotor stimulants are uncorrelated behaviors.
The low (0.3 mg/kg) and high (1.0 mg/kg) doses increased locomotor activity above the saline-treated rats, and the high dose increased locomotor activity above the low dose. In the challenge session the repeatedly-treated amphetamine rats failed to demonstrate increased locomotor activity above the previously saline-treated rats. This null effect was observed across the low and high dose of amphetamine, and suggests that true locomotor sensitization was not observed. The research methods used in the present study are widely used to induce locomotor sensitization. The doses in the present study were chosen to maximally activate individual differences in NSS and USV. The low dose was not sufficient to induce locomotor sensitization, but was chosen because the greatest differences in high and low responders are observed at that dose (Hooks et al. 1992). We also had a relatively low sample size in the acute amphetamine conditions and this could have impacted our ability to observe true sensitization. Importantly, we did observe an increase in locomotor activity between sessions 1 and 7 for the high dose, indicating that amphetamine-induced hyperactivity did increase with repeated amphetamine exposure.
With repeated exposure to psychomotor stimulants 50 kHz USV can sensitize. Sensitization occurs with an overall increase in the total number of USV (Brudzynski et al. 2011b) or a shift from flat USV to FM or trill USV (Ahrens et al. 2009; Ahrens et al. 2013; Taracha et al. 2014). A number of studies have determined that USV undergo a rapid sensitization when administered psychomotor stimulants intravenously (Ahrens et al. 2009). A two injection protocol (TIPS) does not induce USV sensitization, likely suggesting that systemic administration is slower and may be a different process (Taracha et al. 2012). More likely, TIPS sensitization protocol is specific to high vocalizing rats, indicating individual differences in initial amphetamine response is important for the development of USV sensitization (Taracha et al. 2014). The overwhelming evidence from this research and previously cited works indicates that locomotor and affective sensitization are distinct behaviors and warrants additional research to understand how these processes may be related to addictive-like behavior or drug response (Ahrens et al. 2013; Taracha et al. 2014).
The H-USV test uniquely predicted treatment-induced FM USV throughout the duration of the experiment. This suggests that FM USV observed during the H-USV test are a reliable measure that persists throughout repeated drug training. The persistence of the H-USV test predicting FM USV throughout the drug training is in contrast to the NPP test predicting locomotor activity acutely, suggesting that some individual difference traits are important early in drug exposure and diminish, while other traits persist throughout prolonged drug exposure.
NSS may be important trait that moderates drug response early in drug exposure. Later, after prolonged exposure an affective response to the drug may be a more reliable predictor as evidenced by the strengthened interaction between the IEN test and H-USV test after repeated drug exposure. Although NPP did not show any relationship with treatment-induced FM USV, the response to the IEN test changed the H-USV test’s predictive relationship with treatment-induced FM USV. Early in drug training, the IEN test response has little influence on FM USV in response to the respective treatment. However, the influence of the IEN response became stronger with repeated treatments, specifically at the high dose. After repeated treatments, when the IEN response was high, the H-USV relationship was marginally positive. Most importantly, when the IEN response was low, the H-USV test’s predictive validity was much stronger, suggesting that low novelty responders found the high dose more rewarding than high novelty responders. This was only observed in the high dose and not after repeated administrations of the low dose of amphetamine or saline. This interactive relationship was strengthened after a 14-day withdrawal from the drug and provides evidence that the response to novelty moderates affective/motivational response to amphetamine.
This result is consistent with research that suggests that NSS may be a trait that is implicated in compulsive drug taking. Early in drug taking, the NSS trait may not change the reinforcing value of a drug and higher and lower NSS rats experience it similarly. However, after prolonged exposure, the reinforcing value of the drug may be changed differently in higher and lower NSS rats. For lower NSS rats, the reinforcing value of the drug is unchanged and remains a sufficient reinforcer, but in higher NSS rats the reinforcing value of the drug is blunted, but only after repeated exposure. This is important, because it provides evidence that higher NSS rats may experience tolerance faster, and require more drug to experience a reinforcing effect, which correlates with escalation of intake and a behavioral marker of drug dependence (Piazza and Deroche Gamonet. 2013). This is in corroboration with recent research suggesting that high vocalizers in the initial response to amphetamine develop tolerance (Taracha et al. 2014). Other research has also identified high NPP rats as more susceptible to transition to compulsive drug taking (Belin et al. 2011). Therefore, the transition to compulsive drug taking is likely moderated by NSS and provides evidence that the transition to drug dependence in higher NSS rats may be due to a blunted amphetamine value driven by tolerance.
FM 50 kHz USV were dose-dependently changed by amphetamine and NSS; albeit negatively. During session 1 higher vocalizing rats called less when administered the low dose, suggesting the initial affective response was lower. After repeated administrations the low dose did not change FM USV but the high dose decreased FM USV from session 1 to session 7, likely suggesting tolerance (Taracha et al. 2014). This finding is different from other findings (Ahrens et al. 2009; Taracha et al. 2012), but those studies either used intravenous self-administration or only observed FM USV sensitization in high USV callers and not low USV callers. This failure to see similar relationships is likely not due to the use of regression because regression and ANOVA are derived from the General Linear Model and yield comparable results. Regression is appropriate when independent variables are continuous because regression reduces the introduction of error by experimenter-created groups, making regression the more powerful analysis for continuous variables (De Coster et al. 2011). Another explanation for amphetamine’s failure to dose-dependently increase FM 50 kHz USV is that the number of FM 50 kHz USV in response to saline was high. The average number of calls for the saline-treated rats was 254 and 283 for sessions 1 and 7, respectively. This number is considerable higher than other published literature. Although the experimenters provided contextually distinct environments for the H-USV test and locomotor sessions, it is possible that the rats failed to discriminate the environments. Interestingly, if this is true, it would provide strong support that the incentive value from the H-USV test was attributed to the context (Garcia et al. 2015). Furthermore, it would suggest that the saline-treated rats did not extinguish their anticipatory USV response even after 7 sessions without tickling. Therefore, future research needs to explore the relationship between FM 50 kHz USV, contextual conditioning, and incentive salience to determine if the affective response to rewarding stimuli is more resistant to extinction procedures.
The current study demonstrated that both NSS and FM 50 USV are important for understanding rat drug response, albeit separate responses. It appears the choice to engage a novel environment may be important early in psychostimulant abuse, and the response to forced novelty likely moderates the affective response, as measured with FM USV in a dose dependent manner. This provides evidence that the subjective rewarding value of stimulant drugs may be differently assessed in low and high novelty responders, and supports that NSS generally, promotes addictive-like behavior during early and late amphetamine exposure.
Acknowledgments
EJG was supported by 3R15DA035435-01S1.
Abbreviations
- NSS
Novelty and sensation seeking
- IEN
Inescapable novelty test
- NPP
Novelty place preference test
- H-USV
Heterospecific test i.e. tickling
- FM
Frequency modulated
- CPP
Conditioned place preference
Footnotes
The authors do not have any conflicts of interests that would confound the interpretation of the research or data presented.
The authors would like to thank Kathryn C. Johns, Talus J. McCowan, Richard Turner & Dr. Don Saucier for their contribution to the experimental procedures.
References
- Ahrens AM, Nobile CW, Page LE, Maier EY, Duvauchelle CL, Schallert T. Individual differences in the conditioned and unconditioned rat 50-kHz ultrasonic vocalizations elicited by repeated amphetamine exposure. Psychopharmacology (Berl) 2013;229:687–700. doi: 10.1007/s00213-013-3130-9. [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens AM, Ma ST, Maier EY, Duvauchelle CL, Schallert T. Repeated intravenous amphetamine exposure: rapid and persistent sensitization of 50-kHz ultrasonic trill calls in rats. Behav Brain Res. 2009;197:205–209. doi: 10.1016/j.bbr.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt DL, Arnold JC, Cain ME. The effects of mGluR2/3 activation on acute and repeated amphetamine-induced locomotor activity in differentially reared male rats. Exp Clin Psychopharmacol. 2014;22:257–65. doi: 10.1037/a0035273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev. 2013;65:255–90. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Simmons SJ, Servilio LC, Bercovicz D, Ma S, Root DH, Pawlak AP, West MO. Ultrasonic vocalizations: evidence for an affective opponent process during cocaine self-administration. Psychopharmacology (Berl) 2014;231:909–918. doi: 10.1007/s00213-013-3309-0. [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Root DH, Ma S, Jha S, Megehee L, Pawlak AP, West MO. Dose-dependent differences in short ultrasonic vocalizations emitted by rats during cocaine self-administration. Psychopharmacology (Berl) 2010;211:435–442. doi: 10.1007/s00213-010-1913-9. 10.1007/s00213-010-1913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Marusich JA, Gipson CD, Bardo MT. Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behav Brain Res. 2011;216:159–165. doi: 10.1016/j.bbr.2010.07.022. 10.1016/j.bbr.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology. 2011;36:569–579. doi: 10.1038/npp.2010.188. 10.1038/npp.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Agullana R, Weiss SM. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol Behav. 1991;50:967–72. doi: 10.1016/0031-9384(91)90423-l. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Gibson B, Silkstone M, Burgdorf J, Kroes RA, Moskal JR, Panksepp J. Motor and locomotor responses to systemic amphetamine in three lines of selectively bred Long-Evans rats. Pharmacol Biochem Behav. 2011a;100:119–124. doi: 10.1016/j.pbb.2011.08.006. 10.1016/j.pbb.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Silkstone M, Komadoski M, Scullion K, Duffus S, Burgdorf J, Kroes RA, Moskal JR, Panksepp J. Effects of intraaccumbens amphetamine on production of 50 kHz vocalizations in three lines of selectively bred Long-Evans rats. Behav Brain Res. 2011b;217:32–40. doi: 10.1016/j.bbr.2010.10.006. 10.1016/j.bbr.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J. Neurobiology of 50-kHz ultrasonic vocalizations in rats: electrode mapping, lesion, and pharmacology studies. Behav Brain Res. 2007;182:274–283. doi: 10.1016/j.bbr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J, Brudzynski SM, Kroes R, Moskal JR. Breeding for 50-kHz positive affective vocalization in rats. Behav Genet. 2005;35:67–72. doi: 10.1007/s10519-004-0856-5. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J, Ikemoto S. Nucleus accumbens amphetamine microinjections unconditionally elicit 50-kHz ultrasonic vocalizations in rats. Behav Neurosci. 2001;115:940–944. doi: 10.1037//0735-7044.115.4.940. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J. Tickling induces reward in adolescent rats. Physiol Behav. 2001;72:167–173. doi: 10.1016/s0031-9384(00)00411-x. DOI: S0031-9384(00)00411-X [pii]. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J. Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behav Neurosci. 2000;114:320–327. [PubMed] [Google Scholar]
- Cain ME, Coolon RA, Gill MJ. The contribution of the central nucleus of the amygdala to individual differences in amphetamine-induced hyperactivity. Behav Brain Res. 2009;202:11–18. doi: 10.1016/j.bbr.2009.03.007. 10.1016/j.bbr.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain ME, Saucier DA, Bardo MT. Novelty seeking and drug use: contribution of an animal model. Exp Clin Psychopharmacol. 2005;13:367–375. doi: 10.1037/1064-1297.13.4.367. [DOI] [PubMed] [Google Scholar]
- Cain ME, Smith CM, Bardo MT. The effect of novelty on amphetamine self-administration in rats classified as high and low responders. Psychopharmacology (Berl) 2004;176:129–138. doi: 10.1007/s00213-004-1870-2. [DOI] [PubMed] [Google Scholar]
- Coffey KR, Barker DJ, Ma S, Root DH, Martinez L, Horvitz JC, West MO. Effects of varying reinforcement probability on pavlovian approach behavior and ultrasonic vocalizations in rats. Behav Brain Res. 2013;237:256–262. doi: 10.1016/j.bbr.2012.09.041. 10.1016/j.bbr.2012.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Vocalizations during withdrawal from opiates and cocaine: possible expressions of affective distress. Eur J Pharmacol. 2003;467:1–13. doi: 10.1016/s0014-2999(03)01558-9. [DOI] [PubMed] [Google Scholar]
- De Coster J, DeCoster M, Gallucci A, Iselin Best Practices for Using Median Splits, Artificial Categorization, and their Continuous Alternatives. Journal of experimental psychopathology. 2011;2:197–209. [Google Scholar]
- Garcia E, Garcia T, McCowan M, Cain Harmonic and frequency modulated ultrasonic vocalizations reveal differences in conditioned and unconditioned reward processing. Behav Brain Res. 2015 doi: 10.1016/j.bbr.2015.03.049. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Neill DB, Justice JB. Individual differences in amphetamine sensitization: dose-dependent effects. Pharmacology, biochemistry and behavior. 1992;41:203–10. doi: 10.1016/0091-3057(92)90083-r. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB. Individual differences in locomotor activity and sensitization. Pharmacol Biochem Behav. 1991;38:467–70. doi: 10.1016/0091-3057(91)90308-o. [DOI] [PubMed] [Google Scholar]
- Irwin J, Irwin G, McClelland Negative Consequences of Dichotomizing Continuous Predictor Variables. J Market Res. 2003;40:366–371. [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J Comp Psychol. 1998;112:65–73. doi: 10.1037/0735-7036.112.1.65. [DOI] [PubMed] [Google Scholar]
- Ma ST, Maier EY, Ahrens AM, Schallert T, Duvauchelle CL. Repeated intravenous cocaine experience: development and escalation of pre-drug anticipatory 50-kHz ultrasonic vocalizations in rats. Behav Brain Res. 2010;212:109–114. doi: 10.1016/j.bbr.2010.04.001. 10.1016/j.bbr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier EY, Abdalla M, Ahrens AM, Schallert T, Duvauchelle CL. The missing variable: ultrasonic vocalizations reveal hidden sensitization and tolerance-like effects during long-term cocaine administration. Psychopharmacology (Berl) 2012;219:1141–1152. doi: 10.1007/s00213-011-2445-7. 10.1007/s00213-011-2445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews IZ, Morrissey MD, McCormick CM. Individual differences in activity predict locomotor activity and conditioned place preference to amphetamine in both adolescent and adult rats. Pharmacol Biochem Behav. 2010;95:63–71. doi: 10.1016/j.pbb.2009.12.007. 10.1016/j.pbb.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Maxwell S, Maxwell H, Delaney Bivariate median splits and spurious statistical significance. Psychol Bull. 1993;113:181–190. [Google Scholar]
- Meyer PJ, Ma ST, Robinson TE. A cocaine cue is more preferred and evokes more frequency-modulated 50-kHz ultrasonic vocalizations in rats prone to attribute incentive salience to a food cue. Psychopharmacology (Berl) 2012;219:999–1009. doi: 10.1007/s00213-011-2429-7. 10.1007/s00213-011-2429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA. Withdrawal from a self-administered or non-contingent cocaine binge: differences in ultrasonic distress vocalizations in rats. Psychopharmacology (Berl) 1998;136:402–408. doi: 10.1007/s002130050584. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Cuthill Effect size, confidence interval and statistical significance: a practical guide for biologists. Biological reviews. 2007;82:591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- Nathans LL. Interpreting multiple linear regression: A guidebook of variable importance. Practical assessment, research & evaluation. 2012;17:1. [Google Scholar]
- Piazza PV, Deminiere JM, Maccari S, Mormede P, Le Moal M, Simon H. Individual reactivity to novelty predicts probability of amphetamine self-administration. Behav Pharmacol. 1990;1:339–345. doi: 10.1097/00008877-199000140-00007. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza P, Deroche Gamonet V. A multistep general theory of transition to addiction. Psychopharmacology (Berl) 2013;229:387–413. doi: 10.1007/s00213-013-3224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenburg AJ, Honig WM, Van Rossum JM. Inhibition of d-amphetamine-induced locomotor activity by injection of haloperidol into the nucleus accumbens of the rat. Psychopharmacologia. 1975;41:87–95. doi: 10.1007/BF00421062. [DOI] [PubMed] [Google Scholar]
- Sadananda M, Natusch C, Karrenbauer B, Schwarting RK. 50-kHz calls in rats: effects of MDMA and the 5-HT(1A) receptor agonist 8-OH-DPAT. Pharmacol Biochem Behav. 2012;101:258–264. doi: 10.1016/j.pbb.2012.01.012. 10.1016/j.pbb.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Simola N, Fenu S, Costa G, Pinna A, Plumitallo A, Morelli M. Pharmacological characterization of 50-kHz ultrasonic vocalizations in rats: comparison of the effects of different psychoactive drugs and relevance in drug-induced reward. Neuropharmacology. 2012;63:224–234. doi: 10.1016/j.neuropharm.2012.03.013. 10.1016/j.neuropharm.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Simola N, Ma ST, Schallert T. Influence of acute caffeine on 50-kHz ultrasonic vocalizations in male adult rats and relevance to caffeine-mediated psychopharmacological effects. Int J Neuropsychopharmacol. 2010;13:123–132. doi: 10.1017/S1461145709990113. 10.1017/S1461145709990113. [DOI] [PubMed] [Google Scholar]
- Taracha E, Kaniuga S, Chrapusta P, Maciejak L, Sliwa A, Hamed P, Krzascik Diverging frequency-modulated 50-kHz vocalization, locomotor activity and conditioned place preference effects in rats given repeated amphetamine treatment. Neuropharmacology. 2014;83:128–136. doi: 10.1016/j.neuropharm.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Taracha E, Hamed A, Krzascik P, Lehner M, Skorzewska A, Plaznik A, Chrapusta SJ. Inter-individual diversity and intra-individual stability of amphetamine-induced sensitization of frequency-modulated 50-kHz vocalization in Sprague-Dawley rats. Psychopharmacology (Berl) 2012;222:619–632. doi: 10.1007/s00213-012-2658-4. 10.1007/s00213-012-2658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Poel AM, Noach EJ, Miczek KA. Temporal patterning of ultrasonic distress calls in the adult rat: effects of morphine and benzodiazepines. Psychopharmacology (Berl) 1989;97:147–148. doi: 10.1007/BF00442236. [DOI] [PubMed] [Google Scholar]
- Wright JM, Deng L, Clarke PB. Failure of rewarding and locomotor stimulant doses of morphine to promote adult rat 50-kHz ultrasonic vocalizations. Psychopharmacology (Berl) 2012;224:477–487. doi: 10.1007/s00213-012-2776-z. 10.1007/s00213-012-2776-z. [DOI] [PubMed] [Google Scholar]
- Wright JM, Gourdon JC, Clarke PB. Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: effects of amphetamine and social context. Psychopharmacology (Berl) 2010;211:1–13. doi: 10.1007/s00213-010-1859-y. 10.1007/s00213-010-1859-y. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Sensation seeking and the endogenous deficit theory of drug abuse. NIDA Res Monogr. 1986;74:59–70. [PubMed] [Google Scholar]