Figure 1.
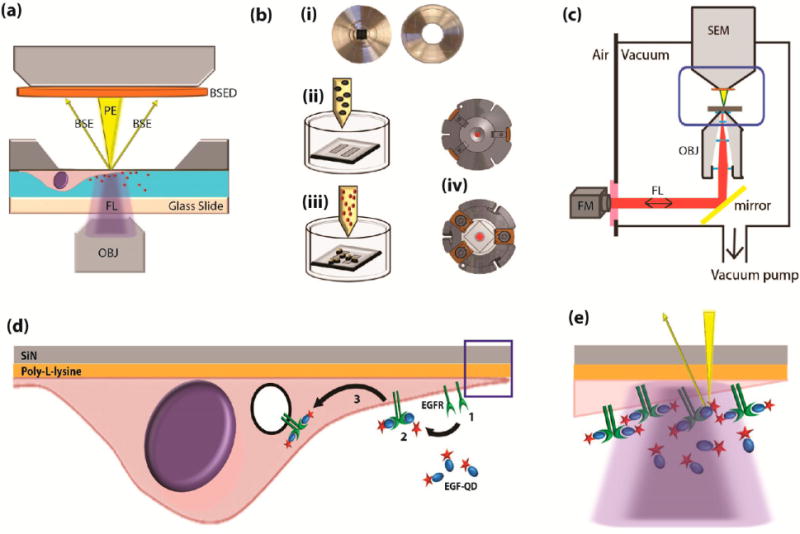
Integrated fluorescence and scanning electron microscopy with live-cell bionanoreactor (a) Schematic of a bionanoreactor for keeping live cells in an integrated fluorescence and scanning electron microscope. Cells are kept in liquid separated from the SEM vacuum by an electron-transparent silicon-supported SiN membrane (dark gray) and a light-transparent glass slide. OBJ, objective lens; FL, fluorescence excitation and emission; PE, primary electron beam; BSE, backscattered electrons; BSED, BSE detector. (b) Procedure for culturing cells and mounting the bionanoreactor: (i) The silicon microchip and the glass slide are glued to separate aluminum plates; (ii) the plate containing the microchip is coated with a thin layer of poly-L-lysine, placed in a Petri-dish and cell culture medium is added; (iii) after cells adhered to the membranes, reagent (EGF-conjugated quantum dots) are added; (iv) the two metal plates are then clamped together in a vacuum-tight holder. (c) Schematic of the integrated fluorescence and scanning electron microscope, with the holder placed in between both microscopes. Blue boxed area corresponds to (a). FM, fluorescence microscope; SEM, scanning electron microscope. (d) Illustration of EGF-mediated uptake of quantum dots (QDOT) in a cell cultured on the SiN membrane. (1) EGF receptors (EGFR) are present on the cell membrane. (2) EGFR dimerize upon binding the EGF-QDOT. (3) The EGFR are taken up and further transported in endosomes within the cell. (e) Particularly high concentration of EGFR occur at tips of cellular extensions (boxed area from (d)), within the light diffraction limit (purple), but discernible with electron microscope resolution (yellow, not to scale).
