Figure 5. Mule tyrosine phosphorylation contributes to its E3 ligase activity.
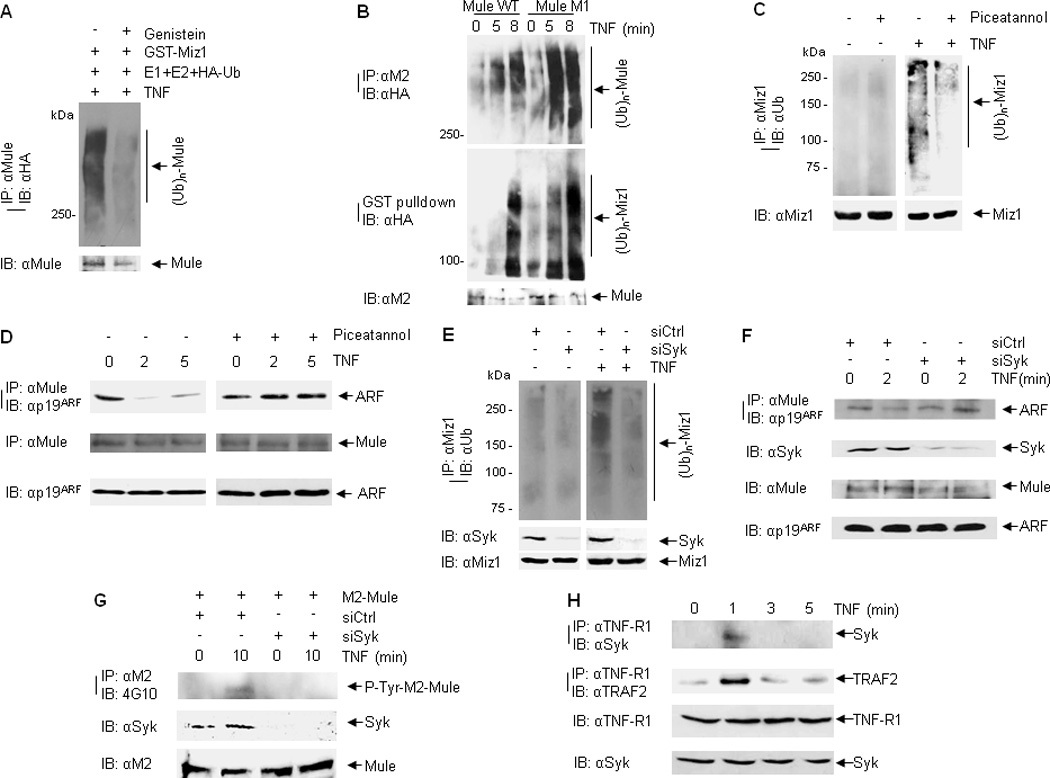
(A) In vitro self-ubiquitination of immunoprecipitated Mule from TNF-treated cells (without or with Genistein pre-treatment). (B) HEK293 cells were transfected with M2-Mule(WT) or M2-Mule(M1) plasmids, followed by stimulation with TNF for various times as indicated. M2-Mule was immunoprecipitated and used for the E3 ligase in an in vitro ubiquitination assay in the absence or presence of GST-Xpress-Miz1. Self-ubiquitination of Mule was analyzed by immunoblotting with HA (ubiquitin) antibody. Ubiquitination of GST-Xpress-Miz1 was determined by GST pulldown followed by immunoblotting with HA (ubiquitin) antibody. (C and D) WT MEFs were pre-treated without or with Piceatannol followed by TNF treatment. Ubiquitination of endogenous Miz1 was determined by immunoprecipitation with Miz1 antibody followed by immunoblotting with ubiquitin antibody (C). Mule-ARF interaction was analyzed by immunoprecipitation with Mule antibody followed by immunoblotting with ARF antibody (D). (E) In vivo ubiquitination of endogenous Miz1 from WT MEFs transfected with control siRNA or Syk siRNA followed by TNF treatment. (F) Mule-ARF interaction from WT MEFs transfected with control siRNA or Syk siRNA followed by TNF treatment. (G) WT MEFs cotransfected with M2-Mule and siRNA (control siRNA or siRNA for ARF) were pretreated with Okadaic Acid and Na3VO4, followed by treatment with TNF for various times as indicated. M2-mule was immunoprecipitated by M2 antibody, followed by immunoblotting with phospho-tyrosine antibody (4G10). (H) WT MEFs were treated with TNF for various times as indicated. The interaction between TNF-R1 and Syk was analyzed by immunoprecipitation with TNF-R1 antibody, followed by immunoblotting with Syk antibody.
