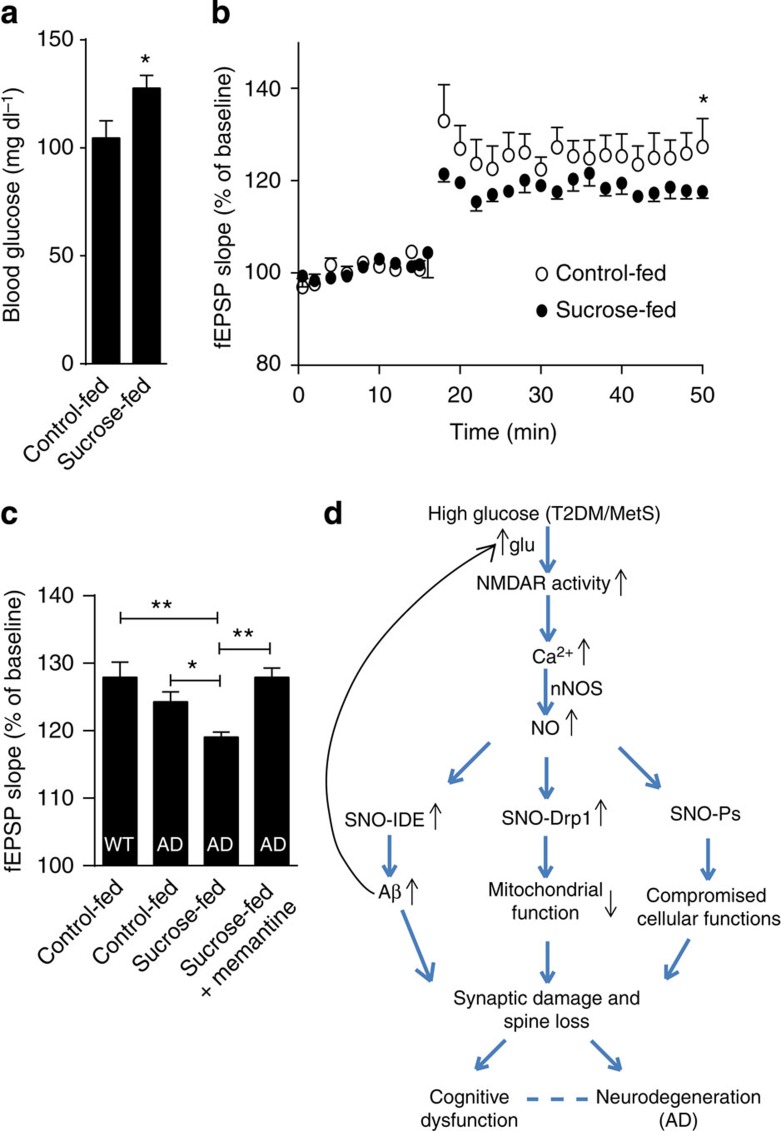
Figure 6. High glucose enhances LTP deficits in the 3 × Tg-AD mouse model.
(a) Sucrose-fed 3 × Tg AD mice show a significant increase in blood glucose levels compared to controls. Values are mean+s.e.m., n=10, P<0.05 by Student's t-test. (b) Hippocampal LTP in 3 × Tg-AD mice. Initial slope of field excitatory postsynaptic potentials (fEPSP) in CA1 pyramidal cells was measured after stimulation of the Schaffer collaterals. Sucrose-fed 3 × Tg-AD mice displayed a significant decrease in LTP compared with controls. (c) Percent potentiation of fEPSP over baseline at 50 min after first tetanus in control (WT) and 3 × Tg-AD mice fed with water containing sucrose, sucrose+0.01% memantine (calculated to provide a dose of 2 mg per kg per day), or normal water. Values are mean+s.e.m., n≥5 mice per condition; *P<0.05, **P<0.01, by ANOVA with Fisher's PLSD post hoc test. (d) Schematic of high glucose and Aβ-induced aberrant S-nitrosylation mechanisms linking T2DM/MetS to AD. High glucose and oligomeric Aβ lead to increased glutamate (glu) release, stimulation of NMDARs, and increased influx of neuronal Ca2+ with consequent NO production. The resulting aberrant S-nitrosylation of IDE leads to increased levels of Aβ and insulin, and aberrant S-nitrosylation of Drp1 contributes to mitochondrial fragmentation and bioenergetic compromise. These redox-mediated changes, potentially among others (designated SNO-Ps in the figure, indicating other S-nitrosylated proteins), culminate in synaptic loss and neurodegeneration, compromising cognitive function in Alzheimer's disease (AD).