Figure 4.
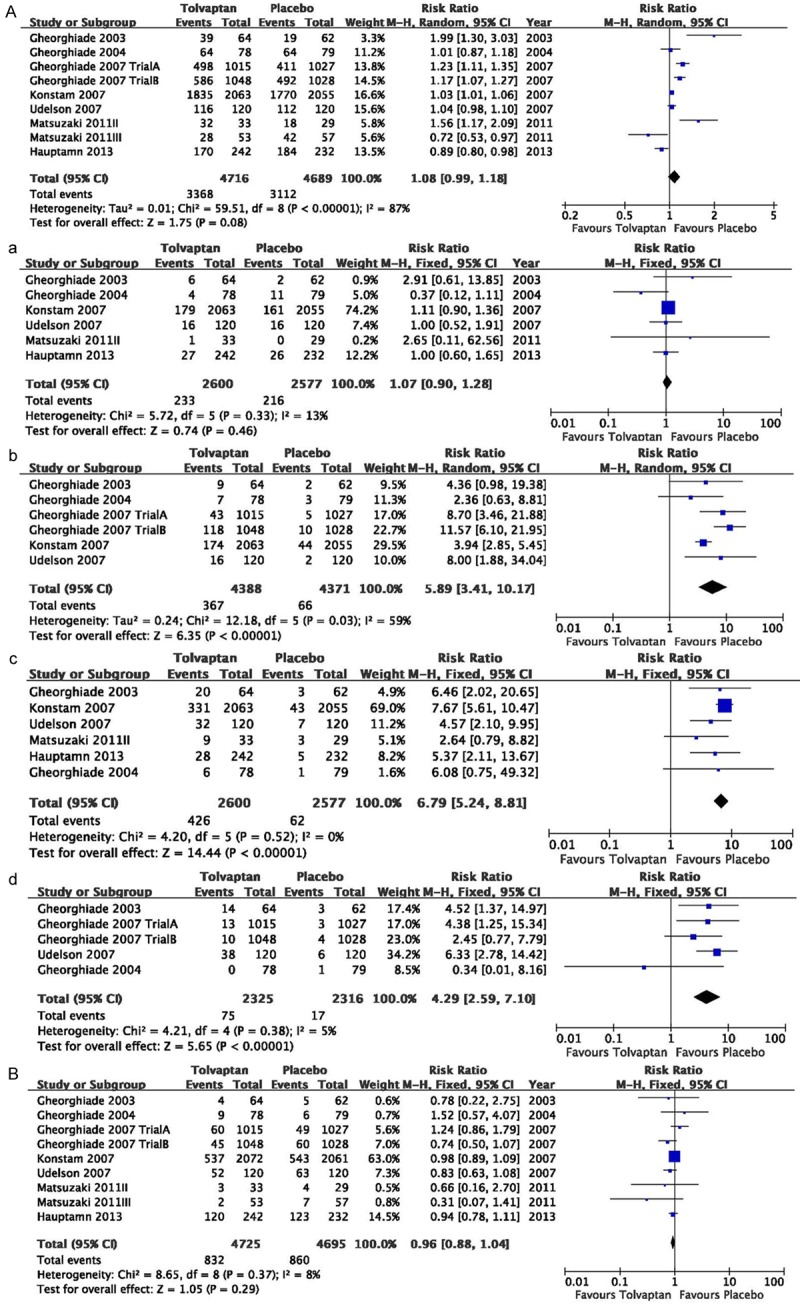
Pooled relative risk in the outcome of common adverse event (A), serious adverse event (B) in the studies considering tolvaptan compared to placebo therapy in HF patients. In the outcome of common adverse event (RR=1.08, 95% CI=0.99 to 1.18, P=0.08) and serious adverse (RR=0.96, 95% CI=0.88 to 1.04, P=0.29) event both show no significant occurrence. In the common adverse event of dizziness (a), dry mouth (b), thirst (c), urinary frequency (d), A significant occurrences were found in dry mouth (RR=5.89, 95% CI=3.41 to 10.17, P<0.001), thirst (RR=6.79, 95%CI=5.24 to 8.81, P<0.001), urinary frequency (RR=4.29, 95% CI=2.59 to 7.10, P<0.001), but dizziness (RR=1.07, 95% CI=0.90 to 1.28, P=0.46).
