Abstract
Objective: This study aimed to compare the therapeutic efficacy of transplantation of human umbilical cord mesenchymal stem cells (hUCMSC) in different routes in acute hepatic failure (ALF) in rats. Methods: hUCMSCs were isolated and identified by detection of surface antigens via flow cytometry. In T group and H group, ALF rats received hUCMSC transplantation through the tail vein and intrahepatic injection, respectively. In hUCMSC group, healthy rats received hUCMSCs transplantation via the tail vein. In ALF group, rats received injection of normal saline through the tail vein. Results: The TBil and ALT in ALF rats with and without transplantation were significantly higher than in healthy rats (P<0.05). HE staining of the liver showed obvious hepatocyte regeneration and reduced infiltration of inflammatory cells, and liver pathology was improved in T group and H group as compared to ALF group. At 3 d after transplantation, CK18 expression was detectable in both H group and T group. At 1 w and 2 w, the mRNA expressions of CK8, CK18 and AFP in H group and T group were significantly different from those in ALF group (P<0.05). The liver function and differentiation of stem cells were comparable between H group and T group (P>0.05). Conclusion: hUCMSCs transplantation can improve the liver function and promote the liver repair following ALF. hUCMSCs transplantation via tail vein has similar therapeutic efficacy to that through intrahepatic injection.
Keywords: Human umbilical cord mesenchymal stem cells, acute liver failure, transplantation, therapy, rat
Introduction
Acute liver failure (ALF) is a syndrome caused by multiple factors and characterized by massive hepatocyte degeneration and necrosis as well as infiltration of inflammatory cells shortly after injury. ALF has a rapid progression and numerous complications, and its treatment is very difficult, which renders it a disease with high mortality and poor prognosis. To date, ALF has been a refractory disease in clinical practice [1]. Traditional pharmacotherapy is unable to timely exert protective effect when the ALF progresses into end stage, bioartificial liver has not been widely used in clinical practice, and liver transplantation is also limited due to the severe organ shortage [2-4]. Mesenchymal stem cells (MSCs) as a source of seed cells have been paid increasing attention to in the field of cell therapy. MSCs may be induced to differentiate into hepatocyets and alter the tissue microenvironment and immune function via a paracrine dependent manner, which is effective for the tissue repair [5-8]. Thus, stem cells transplantation becomes another promising strategy for the therapy of liver failure following orthotopic liver transplantation. Human umbilical cord is a safe and rich source of stem cells and has been another reliable source of MSCs (hUCMSC) after bone marrow MSCs [9-11]. However, few studies have been conducted to investigate the hUCMSC transplantation in the therapy of ALF. In this study, ALF was induced in rats and hUCMSC transplantation was conducted to investigate the effects of hUCMSC transplantation on the liver pathology and the expressions of hepatocytes related genes and to explore the safety and effectiveness of hUCMSC transplantation in the therapy of ALF as well as the optimal route of transplantation.
Materials and methods
Animals and reagents
Animals
Healthy male SD rats (n=48; specific pathogen free, SPF) weighing 130-180 g were purchased from the Experimental Animal Center of Kunming Medical University. Animals were given ad libitum access to food and water. All the procedures were conducted according to the Guide for the Care and Use of Laboratory Animals and this study approved by the Ethics Committee of Kunmin Medical University.
Reagents
Modified Eagle medium-LG (EMEM-LG), fetal bovine serum (FBS), trypsin (HyClone, USA), rabbit anti-human CK18 monoclonal antibody, horseradish peroxidase (HRP) conjugated goat anti-rabbit IgG (Sigma, USA), diaminobenzidine (DAB) substrate kit (Invitrogen, USA), reverse transcription kit (Beijing Bioteke Biotech Co., Ltd), primers (Invitrogen, USA) and Syber fluorescence quantification kit (BD company, USA) were used in the present study.
Separation, culture and identification of hUCMSCs
hUCMSCs were separated and cultured according to previously reported [12]. Cells of the third generation were digested with 0.25% trypsin and re-suspended at a density of 1×106/ml. These cells were independently incubated with following mouse anti-human antibody: FITC-A conjugated CD90 monoclonal antibody, PE-A conjugated CD73 monoclonal antibody, FITC-A conjugated CD45 monoclonal antibody, and PE-A conjugated CD34 monoclonal antibody. Incubation was conducted at room temperature for 30 min in dark. After washing in PBS, cells were subjected to flow cytometry for the detection of these markers. After identification, cells were harvested and re-suspended at a density of 3-5×106/ml for further use.
Establishment of ALF animal model and grouping
ALF was induced by intraperitoneal injection of 50% CCl4 in olive oil at 2.5 ml/kg [13]. Rats developed listlessness, reduced activities, poor appetite, diarrhea, and ascites which are characteristics of liver failure. At 24 h after establishment of animal model, serum ALT and TBil increased significantly, and pathological examination of the liver also revealed the animal model was successfully established.
A total of 48 SD rats were randomly assigned into 4 groups (n=12 per group). (1) Transplantation via tail vein: ALF was induced in rats which received hUCMSCs (3-5×106/ml; 1 ml) transplantation via the tail vein at 24 h after establishment of animal model (T group); (2) Transplantation via intrahepatic injection: ALF was induced in rats which received hUCMSCs (3-5×106/ml; 1 ml) transplantation via the intrahepatic injection (H group); (3) ALF group: ALF was induced in rats which received injection of normal saline (1 ml) via the tail vein; (4) MSCs group: healthy rats received hUCMSCs (3-5×106/ml; 1 ml) transplantation via the tail vein.
Sample collection and detections
At 0 h (24 h after establishment of animal model), 24 h, 48 h, 72 h, 96 h and 1 w after hUCMSCs transplantation, venous blood (1 ml) was collected from the posterior orbital vein and centrifuged 1 h later. The serum was collected into a new EP tube and stored at 4°C for further detections. At 3 d, 1 w and 2 w after hUCMSCs transplantation, 2 rats in each group were sacrificed, and the liver was rapidly collected into a storage tube. Liver tissues were immediately frozen in liquid nitrogen and then stored at -80°C for further detections. At the same time, the right lobe of the liver was collected (about 0.5 cm×1 cm) and fixed in 40 g/L paraformaldehyde.
Serum ALT and TBil
Serum ALT and TBil were measured with the HITACHI 7600 automated biochemistry analyzer.
Pathological examination of the liver
Liver tissues were fixed in paraformaldehyde for 24-48 h, dehydrated in ethanol, treated in xylene, embedded in paraffin and cut into 5-μm sections, followed by HE staining. After mounting, sections were observed under a light microscope and pathological examination was conducted by two experienced pathologists according to the Guideline for the Diagnosis and Therapy of Liver Failure. Consensus was obtained between two pathologists.
Detection of CK18 expression in the liver
Immunohistochemistry was performed to detection CK18 in the liver. Liver sections were heated for 2 d, deparaffinized and dehydrated. After antigen retrieval, sections were incubated with 3% H2O2 at room temperature for 10 min. After washing in PBS, sections were blocked in goat serum, and then incubated with CK18 monoclonal antibody (1:100) at 4°C over night. After incubation with HRP conjugated goat anti-rabbit IgG (1:200) at room temperature for 30 min, anti-protein-peroxidase solution was added, and visualization was conducted with DAB. After counterstaining, fixation, transparentization, and mounting, sections were observed under a light microscope. Fiver fields were randomly selected from each section at a high magnification. CK18 positive cells showed brown granules in the cytoplasm and cell membrane. Image Pro-Plus 6.0 was employed for the detection of optical density (OD) as the CK18 expression.
Detection of CK8, CK18 and AFP mRNA expression
Real-time PCR was conducted to detect the CK8, CK18 and AFP mRNA expressions in the liver according to previously reported [6]. β-actin served as an internal reference, and 2-ΔΔCt method was used to calculate the relative expression of target gene.
Statistical analysis
Statistical analysis was performed with SPSS version 17.0. Quantitative data are expressed as mean ± standard deviation (x̅±s) and compared with two way analysis of variance among groups and Bonferroni method between two groups. The survival rate was compared with Fisher exact test. A value of P<0.05 was considered statistically significant.
Results
Morphology and identification of hUCMSCs
After 3-d culture, cells showed single growth, a few cells were adherent to wall, and most cells were short spindle-shaped. After culture for 7-10 days, cells increased and formed cell monlayer, and most cells were long spindle shaped or polygonal. Cells of the third or forth generation showed favorable morphology and transmittance, became fibroblast-like and displayed whirlpool growth. Flow cytometry showed more than 95% of cells of the third generation were positive for CD90 and negative for CD45, a characteristic of MSCs.
Behaviors and survival rate after ALF
After induction of ALF, rats developed listlessness, reduced activities, poor appetite, and piloerection. Progressively, drowsiness, diarrhea, and ascites, characteristics of liver failure, were present. At 72 h, the survival rate was 16.7% (2/12) in T group, 25.0% (3/12) in H group, 50.0% (6/12) in ALF group and 0 in control group. hUCMSCs transplantation reduced the survival rate as compared to ALF group although significant difference was not observed (P>0.05). In addition, the survival rate was comparable between H group and T group (P>0.05).
Serum ALT and TBil in different groups
At 24 h after ALF, serum ALT and TBil increased significantly, and peaked at 48 h. At 5 d after ALF, the liver function of survived rats gradually returned to nearly normal. At 48 h (24 h after transplantation), 72 h, 96 h and 1 w, the serum ALT and TBil in hUCMSCs transplantation groups were significantly lower than in ALF group (P<0.05). In addition, the serum ALT and TBil in T group were slightly lower than in H group at different time points, although significant difference was not observed (P>0.05; Tables 1 and 2).
Table 1.
Serum TBil at different time points in 4 groups (x±s)
| Group | n | TBil (μmol/L) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Before | 24 h | 48 h | 72 h | 96 h | 1 w | ||
| MSCs | 12 | 0.2±0.1 | 0.3±0.1 | 0.2±0.1 | 0.2±0.2 | 0.2±0.2 | 0.3±0.1 |
| ALF | 12 | 0.3±0.1 | 3.8±1.1 | 7.6±1.3 | 3.8±0.9 | 1.0±0.2 | 0.5±0.2 |
| T | 12 | 0.2±0.1 | 3.8±1.2 | 2.2±1.0a | 1.3±0.4a | 0.6±0.3a | 0.3±0.1a |
| H | 12 | 0.2±0.1 | 4.0±1.0 | 3.0±1.2a | 1.4±0.2a | 0.8±0.3a | 0.3±0.2a |
P<0.05 vs. ALF group.
hUCMSCs transplantation was conducted at 24 h after establishment of animal model. ALF: acute liver failure, T: tail vein transplantation; H: intrahepatic transplantation; MSCs: mesenchymal stem cells.
Table 2.
Serum ALT at different time points in 4 groups (x±s)
| Group | n | ALT(U/L) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Before | 24 h | 48 h | 72 h | 96 h | 1 w | ||
| MSCs | 12 | 36.8±7.4 | 33.4±8.3 | 34.5±3.7 | 40.4±4.8 | 38.5±9.2 | 35.9±6.4 |
| ALF | 12 | 36.3±7.8 | 1157.5±282.4 | 1673.4±399.5 | 495.1±134.2 | 190.6±60.3 | 59.8±10.4 |
| T | 12 | 34.5±6.6 | 1239.7±275.3 | 847.5±202.1a | 148.5±54.6a | 82.5±28.7a | 38.3±10.3a |
| H | 12 | 36.0±9.0 | 1296.2±264.6 | 955.0±266.1a | 197.2±64.2a | 98.0±22.3a | 37.5±11.6a |
P<0.05 vs. ALF group.
hUCMSCs transplantation was conducted at 24 h after establishment of animal model. ALF: acute liver failure, T: tail vein transplantation; H: intrahepatic transplantation; MSCs: mesenchymal stem cells.
Liver pathology of different groups
In MSCs group, there were not hepatocyte degeneration and necrosis. In T group, H group and ALF group, the hepatocyte necrosis expanded outward the hepatic lobules, which was accompanied by infiltration of inflammatory cells and sinus congestion at 24 h, and degenerated hepatocytes were only found at the periphery of hepatic lobules. At 3 d and 1 w, the hepatocyte degeneration was attenuated, cytoplasm was even, cell arrangement was relatively regular, the structure of hepatic lobules was clear, newly generated cells were found along the periportal region, and these cells were large in volume and had large and dark nucleus or even double nuclei in H group and T group. In ALF group, the hepatocyte arrangement was relatively regular, but cells were blurry, degenerated cells were still observed in the peri-portal region, cytoplasm was loose and a large amount of inflammatory cells infiltrated (Figure 1).
Figure 1.
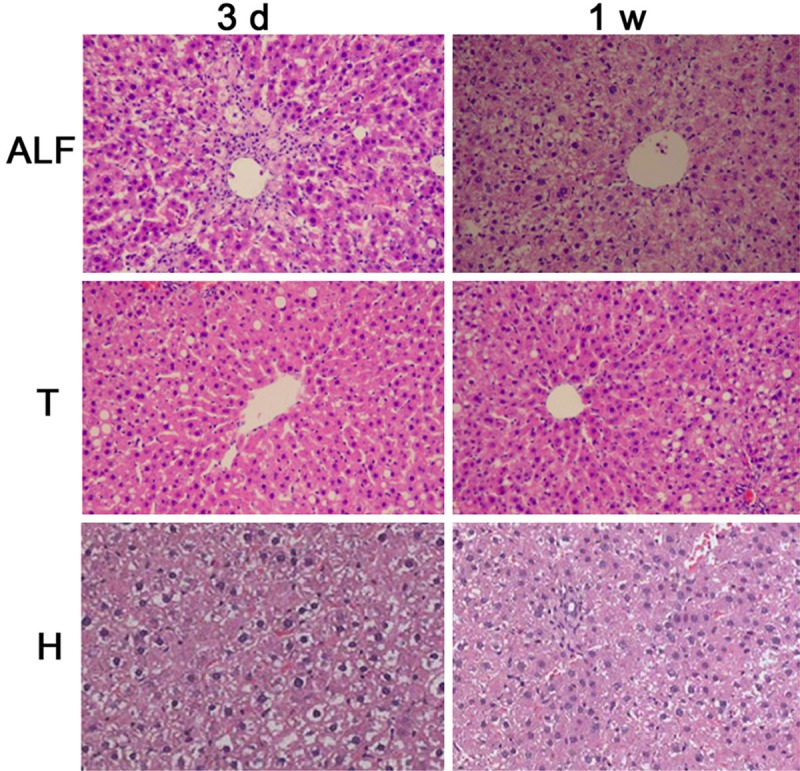
Liver pathology of different groups (HE×100). ALF: acute liver failure, T: tail vein transplantation; H: intrahepatic transplantation.
CK18 protein expression in the liver
At 3 d after transplantation, CK18 protein expression was detected in H group and T group. CK18 was mainly distributed along the peri-portal region. At 1 w and 2 w, cells positive for CK18 migrated into the center of hepatic lobules (Figure 2). However, CK18 was undetectable in ALF group at any time point.
Figure 2.
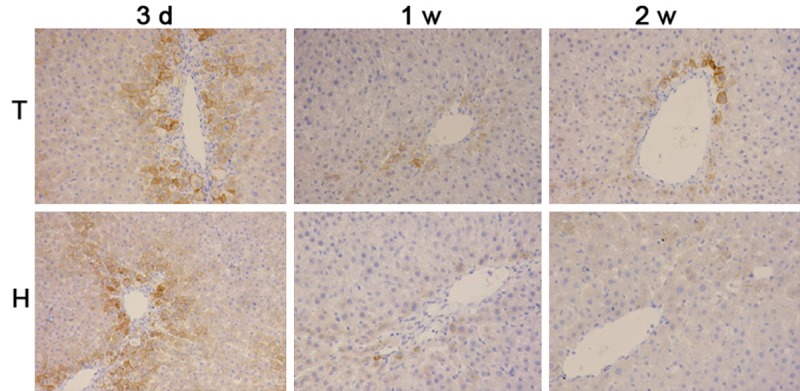
CK18 protein expression in the liver (immunohistochemistry ×100). At 3 d after transplantation, CK18 protein expression was detected in H group and T group. At 1 w and 2 w, cells positive for CK18 migrated into the center of hepatic lobules. ALF: acute liver failure, T: tail vein transplantation; H: intrahepatic transplantation; MSCs: mesenchymal stem cells.
mRNA expressions of CK8, CK18 and AFP in the liver
At 3 days after transplantation, the mRNA expressions of CK8, CK18 and AFP remained at a low level in the liver. At 1 w and 2 w, the mRNA expressions of CK8, CK18 and AFP began to increase and were significantly higher than those before (P<0.05). In addition, the mRNA expressions of CK8, CK18 and AFP in T group were higher than in H group at 3 d, 1 w and 2 w although there was no marked difference (P>0.05; Figures 3 and 4).
Figure 3.
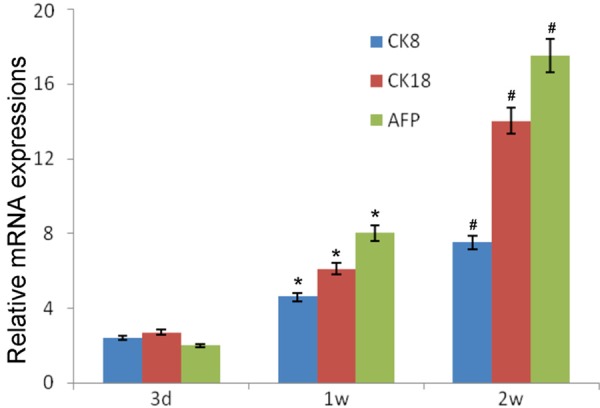
mRNA expressions of CK8, CK18 and AFP in tail vein transplantation group (T group). Compared with time at 3 d, *P<0.05; Compared with time at 1 w, #P<0.05.
Figure 4.
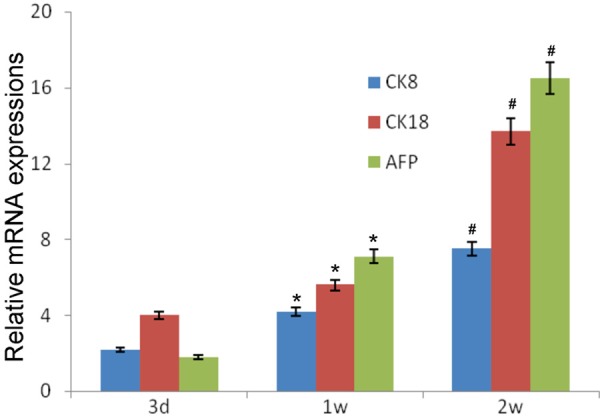
mRNA expressions of CK8, CK18 and AFP in intrahepatic transplantation group(H group). Compared with time at 3 d, *P<0.05; Compared with time at 1 w, #P<0.05.
Discussion
Following ALF, a large amount of hepatocytes becomes necrotic. Under this condition, the endogenous hepatocyte regeneration fails to timely and effectively repair the injured liver, resulting in a high mortality [14]. Exogenous MSCs have potent capabilities of self-proliferation and immunoregulation, which offsets the incomplete repair of endogenous hepatocytes. These MSCs may differentiate into functional hepatocytes and also promote hepatocytes to repair injured liver via paracrine, which is helpful for the attenuation of liver injury [15]. MSCs were first separated from the bone marrow. However, bone marrow collection is invasive, and the number of MSCs separated from bone marrow is small and may not meet the clinical requirement for autologous cell transplantation. Thereafter, investigators found that umbilical cord and cord blood are rich in MSCs which have the pluripotent as in MSCs from bone marrow. MSCs from umbilical cord and cord blood have been successfully used in the therapy of organ failure and immune related diseases [16]. In respect of current techniques in China, the separation and purification of MSCs from umbilical cord blood are very difficult and often expensive, and the quality of these cells may not be guaranteed. However, the separation of MSCs from umbilical cord is relatively simple and has a high success rate, and has been used in several experimental institutes for industrial production in China. MSCs from umbilical cord have the advantages of stable source, reliable quality and easy industrial production. Thus, MSCs from umbilical cord were used in the present study.
MSCs can differentiate into hepatocyte-like cells, which has been confirmed in studies [17]. The prognosis of ALF is mainly dependent on the regeneration of hepatocytes. Under pathological conditions, the liver has a compromised capability to regenerate hepatocytes. In recent years, studies reveal that hUCMSC transplantation may improve the liver failure because the transplanted hUCMSCs may repair the injured liver via paracrine or through differentiating into hepatocyte-like cells [18,19]. In the present study, results showed both intravenous and intrahepatic transplantation could reduce serum ALT and TBil and improve liver pathology. This suggests that hUCMSCs transplantation is able to effectively treat ALF, which was consistent with previously reported.
hUCMSCs may repair the injured liver via pracrine or through differentiating into hepatocyte like cells [20-22]. MSCs can differentiate into hepatocytes, which have been confirmed in studies. There is evidence showing that the phenotypes of liver stem cells and their response in humans are similar to findings observed in animal models in the presence of acute or chronic liver diseases. Tsai et al [23] found the transplanted hUCMSCs were mainly distributed in the connective tissues of the liver, but they did not directly differentiate into hepatocytes, and they confirmed that these stem cells could secrete a lot of bioactive factors to promote the liver repair and hepatocyte regeneration. Ren et al [24] also found that hUCMSCs differentiated into hepatocyte-like cells to improve liver function, but they failed to promote the vascularization of hepatic sinus. They revealed that human specific AFP and ALB mRNAs were detected in the liver of NOD/SCID rats with chronic hepatic fibrosis after hUCMSC transplantation, VEGF expression in the liver increased markedly as compared to normal liver, but the vascular density (CD31 and vWF) remained unchanged. Several studies have confirmed in different (chronic) animal models of liver diseases that MSCs transplantation is able to inhibit inflammation in the liver, promote hepatocyte regeneration, and inhibit the hepatic fibrosis, leading to the improvement of liver function.
However, the adjustability of MSCs proliferation is still controversial [25]. In a study, MSCs passaged for 20 generations have detectable telomerase activity, proto-oncogene c-myc over-expression and inhibition of tumor suppressor genes (Rb, p53 and P16), as well as Wnt signaling pathway down-regulation (all are characteristics of cancer cells), leading to MSCs immortalization. This suggests these cells might have the tumorigenic activity [26]. However, to date, no study has revealed the tumorigenic effect of MSCs after transplantation. On the contrary, MSCs are able to inhibit the tumor formation via secreting IFN-β, IFN-γ, IL-2 and IL-12 or expressing TRAIL. In future studies, it is necessary to control the passaging and quality of hUCMSCs and the molecular and genetic characteristics of hUCMSCs should be maintained, which are crucial for the safe clinical application of hUCMSCs transplantation.
MSCs can be transplanted via different routes including intraperitoneal injection, portal venous injection, hepatic arterial injection, peripheral venous injection, intrasplenic injection and intrahepatic injection [27,28]. Currently, transplantation via the intrahepatic portal vein or hepatic artery is often used, but its operation is difficult and also invasive and has the possibility of causing thrombosis. In addition, transplantation via the hepatic artery is not helpful for the transdifferentiation of stem cells. Thus, transplantation via peripheral veins is more feasible in the clinical therapy of liver diseases [29,30]. Yuan et al [31] compared the therapeutic efficacy of BMSCs transplantation via portal vein as compared to that via tail vein in rats with liver failure. Their results showed the number of MSCs migrating into the liver and the cloning of MSCs were not associated with the routes of transplantation, but related to liver injury. In the present study, MSCs were transplanted via tail vein injection and through intrahepatic injection. Results showed MSCs transplantation via tail vein injection seemed to exert a better therapeutic effect as compared to that through intrahepatic injection. At 1 d after hUCMSCs transplantation, the liver function was improved; at 3 d, human specific markers for hepatocytes were detectable in rats receiving hUCMSCs transplantation in both groups. Cells positive for CK18 were mainly distributed along the peri-portal region, and these cells differentiated into mature cells and migrated into the area of central vein, which is consistent with biological law of hepatocyte regeneration after liver injury.
Acknowledgements
This study was supported by Natural Science Foundation of China (81360072), Yunnan Natural Science Fund (2012FD095), Key Program of Yunnan Provincial Research Fund of Department of Education (2014Z125, 2015Z146) and Yunnan Key Clinical Specialist Construction Project (YWYF[2015]18#).
Disclosure of conflict of interest
None.
References
- 1.Reddy KR, Ellerbe C, Schilsky M, Stravitz RT, Fontana RJ, Durkalski V, Lee WM Acute Liver Failure Study Group. Determinants of Outcome Among Patients with Acute Liver Failure Listed for Liver Transplantation in the US. Liver Transpl. 2015 doi: 10.1002/lt.24347. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minami S, Shibata M, Matsuhashi T, Hiura M, Abe S, Harada M. Acute Liver Failure Complicated with Severe Heart Failure. Intern Med. 2015;54:2443–2447. doi: 10.2169/internalmedicine.54.2913. [DOI] [PubMed] [Google Scholar]
- 3.Guo Y, He Y, Zhang Y, Zhou Y, Qin Y, Fan C, Ji G, Zhang P, Jia Z. Upregulated Expression of A20 on Monocytes is Associated With Increased Severity of Acute-on-Chronic Hepatitis B Liver Failure: A Case-Control Study. Medicine (Baltimore) 2015;94:e1501. doi: 10.1097/MD.0000000000001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim TY, Kim DJ. Acute-on-chronic liver failure. Clin Mol Hepatol. 2013;19:349–359. doi: 10.3350/cmh.2013.19.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CH, Shah B, Moioli EK, Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest. 2015;125:3992. doi: 10.1172/JCI84508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook D, Genever P. Regulation of mesenchymal stem cell differentiation. Adv Exp Med Biol. 2013;786:213–229. doi: 10.1007/978-94-007-6621-1_12. [DOI] [PubMed] [Google Scholar]
- 7.Li M, Ikehara S. Bone-marrow-derived mesenchymal stem cells for organ repair. Stem Cells Int. 2013;2013:132642. doi: 10.1155/2013/132642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bader AM, Klose K, Bieback K, Korinth D, Schneider M, Seifert M, Choi YH, Kurtz A, Falk V, Stamm C. Hypoxic Preconditioning Increases Survival and Pro-Angiogenic Capacity of Human Cord Blood Mesenchymal Stromal Cells In Vitro. PLoS One. 2015;10:e0138477. doi: 10.1371/journal.pone.0138477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vangsness CT Jr, Sternberg H, Harris L. Umbilical Cord Tissue Offers the Greatest Number of Harvestable Mesenchymal Stem Cells for Research and Clinical Application: A Literature Review of Different Harvest Sites. Arthroscopy. 2015;31:1836–1843. doi: 10.1016/j.arthro.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Fazzina R, Mariotti A, Procoli A, Fioravanti D, Iudicone P, Scambia G, Pierelli L, Bonanno G. A new standardized clinical-grade protocol for banking human umbilical cord tissue cells. Transfusion. 2015 doi: 10.1111/trf.13277. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Nan C, Shi Y, Zhao Z, Ma S, Liu J, Yan D, Song G, Liu H. Monosialoteterahexosyl ganglioside induces the differentiation of human umbilical cord-derived mesenchymal stem cells into neuron-like cells. Int J Mol Med. 2015;36:1057–1062. doi: 10.3892/ijmm.2015.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao L, Huang DC, Gong MJ, Li YS, Bi Y. Repair effect of hepatic progenitor cell transplantation on acute liver failure mouse model induced by carbon tetrachloride. J Jilin Univ (Med Ed) 2015;41:463–469. [Google Scholar]
- 14.Frongillo F, Bianco G, Silvestrini N, Lirosi MC, Sanchez AM, Nure E, Gaspari R, Avolio AW, Sganga G, Agnes S. Acute Liver Failure in an Adult, a Rare Complication of Alagille Syndrome: Case Report and Brief Review. Transplant Proc. 2015;47:2179–2181. doi: 10.1016/j.transproceed.2014.11.072. [DOI] [PubMed] [Google Scholar]
- 15.Talaei-Khozani T, Borhani-Haghighi M, Ayatollahi M, Vojdani Z. An in vitro model for hepatocyte-like cell differentiation from Wharton’s jelly derived-mesenchymal stem cells by cell-base aggregates. Gastroenterol Hepatol Bed Bench. 2015;8:188–199. [PMC free article] [PubMed] [Google Scholar]
- 16.Shi LL, Liu FP, Wang DW. Transplantation of human umbilical cord blood mesenchymal stem cells improves survival rates in a rat model of acute hepatic necrosis. Am J Med Sci. 2011;342:212–217. doi: 10.1097/MAJ.0b013e3182112b90. [DOI] [PubMed] [Google Scholar]
- 17.Zheng S, You LY. Research progress in the differentiation of mesenchymal stem cells into functional hepatocytes. Chin J Hepato Surg. 2013;19:396–400. [Google Scholar]
- 18.Lin H, Xu R, Zhang Z, Chen L, Shi M, Wang FS. Implications of the immunoregulatory functions of mesenchymal stem cells in the treatment of human liver diseases. Cell Mol Immunol. 2011;8:19–22. doi: 10.1038/cmi.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alizadeh E, Eslaminejad MB, Akbarzadeh A, Sadeghi Z, Abasi M, Herizchi R, Zarghami N. Upregulation of MiR-122 via Trichostatin A Treatments in Hepatocyte-like Cells Derived from Mesenchymal Stem Cells. Chem Biol Drug Des. 2015 doi: 10.1111/cbdd.12664. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Manzini BM, da Silva Santos Duarte A, Sankaramanivel S, Ramos AL, Latuf-Filho P, Escanhoela C, Kharmandayan P, Olalla Saad ST, Boin I, Malheiros Luzo AC. Useful properties of undifferentiated mesenchymal stromal cells and adipose tissue as the source in liver-regenerative therapy studied in an animal model of severe acute fulminant hepatitis. Cytotherapy. 2015;17:1052–1065. doi: 10.1016/j.jcyt.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Li T, Xia M, Gao Y, Chen Y, Xu Y. Human umbilical cord mesenchymal stem cells: an overview of their potential in cell-based therapy. Expert Opin Biol Ther. 2015;15:1293–1306. doi: 10.1517/14712598.2015.1051528. [DOI] [PubMed] [Google Scholar]
- 22.Yang JF, Cao HC, Pan QL, Yu J, Li J, Li LJ. Mesenchymal stem cells from the human umbilical cord ameliorate fulminant hepatic failure and increase survival in mice. Hepatobiliary Pancreat Dis Int. 2015;14:186–193. doi: 10.1016/s1499-3872(15)60354-x. [DOI] [PubMed] [Google Scholar]
- 23.Tsai PC, Fu TW, Chen YM, Ko TL, Chen TH, Shih YH, Hung SC, Fu YS. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton’s jelly in the treatment of rat liver fibrosis. Liver Transpl. 2009;15:484–495. doi: 10.1002/lt.21715. [DOI] [PubMed] [Google Scholar]
- 24.Ren H, Zhao Q, Cheng T, Lu S, Chen Z, Meng L, Zhu X, Yang S, Xing W, Xiao Y, Ren Q, Chi Y, Gu D, Yang R, Han ZC. No contribution of umbilical cord mesenchymal stromal cells to capillarization and venularization of hepatic sinusoids accompanied by hepatic differentiation in carbon tetrachloride-induced mouse liver fibrosis. Cytotherapy. 2010;12:371–383. doi: 10.3109/14653241003596661. [DOI] [PubMed] [Google Scholar]
- 25.Salem B, Miner S, Hensel NF, Battiwalla M, Keyvanfar K, Stroncek DF, Gee AP, Hanley PJ, Bollard CM, Ito S, Barrett AJ. Quantitative activation suppression assay to evaluate human bone marrow-derived mesenchymal stromal cell potency. Cytotherapy. 2015;17:1675–86. doi: 10.1016/j.jcyt.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzman DL, Antonarakis ES. Clinical Implications of Hedgehog Pathway Signaling in Prostate Cancer. Cancers (Basel) 2015;7:1983–1993. doi: 10.3390/cancers7040871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim G, Eom YW, Baik SK, Shin Y, Lim YL, Kim MY, Kwon SO, Chang SJ. Therapeutic Effects of Mesenchymal Stem Cells for Patients with Chronic Liver Diseases: Systematic Review and Meta-analysis. J Korean Med Sci. 2015;30:1405–1415. doi: 10.3346/jkms.2015.30.10.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Follenzi A, Raut S, Merlin S, Sarkar R, Gupta S. Role of bone marrow transplantation for correcting hemophilia A in mice. Blood. 2012;119:5532–5542. doi: 10.1182/blood-2011-07-367680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilgenkrantz H, Collin de l’Hortet A. New insights into liver regeneration. Clin Res Hepatol Gastroenterol. 2011;35:623–629. doi: 10.1016/j.clinre.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Wang Q, Song WF, Yi SH, Zhang Q, Liu JR, Chen GH. Effect of human umbilical cord-derived mesenchymal stem cells transplantation on ischemic-type biliary lesions rabbit model. Ogran Transplant. 2012;03:127–132. [Google Scholar]
- 31.Yuan SF, Hu LY, Jiang T, Sun LH, Zheng RJ, Zhao JY, Zhang YX. Effect of bone marrow mesenchymal stem cells transplantation on the expression of CD163 and interleukin-10 in rats with acute hepatic liver failure. J Clin Rehab Tiss Engineer Res. 2014:919–925. [Google Scholar]


