Abstract
Objective: This study aimed to map differences in extension patterns between adenoid cystic carcinoma (ACC) of the nasopharynxandnasopharyngeal carcinomaon magnetic resonance imaging (MRI) and provide more information for treatments. Methods: MRI examinations and clinical records were reviewed in 18 patients with ACC of the nasopharynx and 182 patients with nasopharyngeal carcinoma (NPC). All patients had biopsy to confirm diagnosis. Differences between NPC and ACC in terms of extension patterns were identified by the retrospective analysis of images from patients undergoing MRI. Results: Patients with ACC of the nasopharynx obtained a higher rate of staging T4 (14.3% vs. 38.9%, P = 0.007) and paralyzed cranial nerves (6.0% vs. 38.9%, P < 0.001). Epstein-Barr (EB) virus infection was not correlated with ACC incidence. No significant difference was observed in invasion to skull base between ACC of the nasopharynx and NPC (53.3% vs. 66.7%, P = 0.277). Involvement of cranial nerve canal (32.4% vs. 55.6%, P = 0.049) and cavernous sinus (13.7% vs. 33.3%, P = 0.028) was more frequently detected by MRI on patients with ACC of the nasopharynx. Lymph node metastasis was infrequently diagnosed in patients with ACC of the nasopharynx.Conclusion: As seen on MRI images, ACC of the nasopharynx is characterized by a high incidence of perineural invasion, frequent and aggressive local infiltration, and infrequently, lymph node metastasis.
Keywords: Adenoid cystic carcinoma, nasopharyngeal carcinoma, extension patterns, MRI, paralyzed cranial nerve
Introduction
Nasopharyngeal carcinoma (NPC), the most common malignancy of the nasopharynx, is frequently diagnosed in southern China and Hong Kong [1]. Squamous cell carcinoma is the main form of malignancy in primary NPC, which can be classified as keratinizing squamous cell carcinoma and nonkeratinizing carcinoma. The NPC incidence is predicted to be 0.6/100000 population in more developed areas and 2.1/100000 population in less developed areas. The highest incidence rate is found in southern China, with an estimated number of 30/100000 to 50/100000 population [2].
Even in a high-risk NPC area, ACC is a rare tumor entity and forms in approximately 1% of all carcinomas of the head and neck [3]. ACC is classified as a pathological form of adenocarcinoma. ACC of the nasopharynx is even less rarely reported since it was discovered by Billroth in 1856 [4]. Reports on a large number of patients with ACC of the nasopharynx are lacking, which means knowledge about its extension patterns is poor.
Differentiating ACC of the nasopharynx from NPC is difficult. The only way to identify the disease is biopsy which is disadvantageous to clinical diagnosis and treatments. Magnetic resonance imaging (MRI) is a particular method of interest because it depicts perineural infiltration and bone marrow changes by providing satisfactory morphological information of the structures. MRI has been widely used for diagnosing and monitoring the response of treatment for carcinomas of the head and neck. Extensions to the skull base and the deep facial space can be illustrated well using MR images. Lymph node metastases are also evaluated by MRI for TNM staging which tightly correlates with the prognosis. Different biological characters, especially extension patterns, show differences on MR images, which provides the possibility of correctly differentiate the two forms of malignancy by MRI. No previous study on extension patterns of ACC by MRI exists.
The extension patterns on MR images in ACC patients are illustrated using comparisons between common NPC and ACC. Results can provide more information for clinicians to diagnose the disease and to create therapeutic strategies.
Materials and methods
A database search was done to identify ACC patients with confirmed pathological diagnosis between December 2002 and December 2012, and NPC patients with confirmed pathological diagnosis between January 2005 and March 2005 who took MRI examination at our hospital. In total, 18 patients with ACC of the nasopharynx and 182 patients with NPC were recruited. An institutional review board exemption and a waiver of the requirement for written informed consent were obtained to perform this retrospective study.
MRI was performed with a 1.5 T MRI unit (GyroscanIntera, Philips Medical Systems, Best, the Netherlands) and a body coil (Philips Medical Systems, Best, The Netherlands). The head and neck MRI protocol included unenhanced axial, sagittal, and coronal T1-weighted sequences, axial T2-weighted sequences, and contrast-enhanced axial, sagittal, and coronal T1-weighted sequences. The parameters for these sequences were as follows: T1-weighted fast-field echo (FFE) sequence (TR: 500-600; TE = 10-20 ms axial slice thickness, 5.0 mm; axial slice interval, 1.0 mm; coronal and sagittal slice thickness, 6.0 mm; slice interval, 1.0 mm; and matrix scan, 512 × 512); and T2-weighted turbo-spin echo sequence (TR, 4000-6000; TE, 95-110 ms; axial slice thickness, 5.0 mm; axial slice interval, 1.0 mm; coronal and sagittal slice thickness, 6.0 mm; slice interval 1.0 mm; and matrix scan, 512 × 512). Contrast agent at an intravenous dose of 0.1 mmol/kg to 0.2 mmol/kg (Gadolinium-DTPA, magnevist, Schering) was administered to patients after unenhanced T1-weighted and T2-weighted sequences for contrast-enhanced axial and sagittal T1-weighted sequences, and contrast-enhanced, coronal fat-suppressed T1-weighted sequences.
All the images were transferred to the Centricity RIS/PACS workstation (General Electric Healthcare Centricity RIS CE Systems) for post-review. MRI images were analyzed by an experienced radiologist to describe the characteristics of each lesion, including the location, shape, size, number, edge, and attenuation or intensity of the unenhanced and contrast-enhanced lesions [5]. Professional experts reviewed the images with diagnosis of NPC or ACC and results were derived by consensus. On the unenhanced MR images, attenuation or intensity was classified as low, moderate, or high in contrast to adjacent tissues. Conversely, on contrast-enhanced MR images, the enhancement was classified as no, mild, moderate, or marked enhancement [6].
The evaluation criteria for MRI diagnosis were as follows [7]. a. Parapharyngeal space invasion: the lesion exceeded the pharyngobasilar fascia. b. Retropharyngeal lymph node metastases: diameter ≥5 mm, obvious necrosis, or ring hyperintense signal. c. Nasal cavity invasion: the lesion exceeded the line between both sides of pterygopalatine fossa. d. Oropharynx invasion: the lesion exceeded the line along the inferior edge of the anterior arch or through the atlanto-axial articulation. e. Laryngopharynx invasion: the lesion exceeded the upper edge of epiglottis. f. Skull base invasion: Any part of pterygoid process (e.g., medial plate, lateral plate, and pterygoid base), petrous apex, clivus, foramen lacerum, foramen ovale, basisphenoid, or foramen magnum was involved. On MRI, hyperintense image of marrow on T1-weighted images was replaced by isointense image of tumor entity [8]. g. Cranial nerve invasion must meet at least one of the following criteria [9]: (1) Perineural infiltration, soft tissue mass appeared within neural canal on contrast-enhanced T1-weighted images, replacing normal neuromechanism. (2) Tumor spreading along the neural canal, and MRI images showed gross nerves with branches with enhancement and/or enlargement of the neural foramens. Fat tissue was replaced by tumor mass with enhancement as primary tumor mass in neural foramens, including the foramen rotundum, the foramen ovale, the hypoglossal canal, the jugular foramen, the pterygopalatine fossa, and the pterygoid canal. h. TNM classification of ACC and NPC was adapted in 2010, with the seventh edition of the American Joint Committee on Cancer TNM classification [10].
Biopsy was performed under conchoscope to confirm the diagnosis on MRI. The histological techniques included routine hematoxylin and eosin staining and immunohistochemical evaluation. All pathological samples were retrospectively reviewed by two professional pathologists and results were derived by consensus. The macroscopic features of tumor tissues were documented: shape, size, number, edge, and capsule wall. Pathological forms were evaluated according to World Health Organization (WHO) pathological criteria [11].
Finally, we compared MRI images of ACC and NPC and recorded the differences. Archives were assembled for each patient with SPSS 16.0 software. The general clinical data from the two groups (such as tumor situations and complications of hepatitis) were inspected by χ2 test. Values of P < 0.05 were considered statistically significant.
Results
Clinical data
In total, we gathered information on 18 patients with ACC of the nasopharynx and 182 NPC patients. In the NPC group were 136 males (75%) and 46 females (25%) with a mean age of 46.8 years old (in the range 11 years old to 78 years old). Only one patient had pathological type I, whereas the others were type II. In the ACC group were 8 males (44.4%) and 10 females (55.6%) with a mean age of 47.1 years old (in the range 33 years old to 65 years old). Results are displayed in Table 1.
Table 1.
General information in two groups
| Characteristic | ACC patients (n) | NPC patients (n) |
|---|---|---|
| No. of patients | 18 | 182 |
| Mean age (y) | 47.1 | 46.8 |
| Gender | ||
| M | 8 | 136 |
| F | 10 | 46 |
| Serum EBV (n, %) | 3 (16.7) | 164 (90.1) |
| T Stage (n, %) | ||
| T1 | 4 (22.2) | 31 (31.9) |
| T2 | 2 (11.1) | 54 (29.7) |
| T3 | 5 (27.7) | 71 (39.0) |
| T4 | 7 (38.9) | 26 (14.3) |
| N Status (n, %) | ||
| N0 | 16 (88.9) | 63 (34.6) |
| N1 | 1 (5.3) | 65 (35.5) |
| N2 | 1 (5.3) | 37 (20.6) |
| N3 | 0 (0) | 26 (14.3) |
| Staging (n, %) | ||
| I | 4 (22.2) | 25 (13.7) |
| II | 2 (11.1) | 49 (26.9) |
| III | 4 (22.2) | 78 (42.8) |
| IVa-b | 8 (44.4) | 30 (16.5) |
NPC: nasopharyngeal carcinoma; NACC: adenoid cystic carcinoma of the nasopharynx; n: number. y: year; M: Male; F: Femaie; EBV: EB virus.
The data were inspected by χ2 between the two groups. There were statistically significant differences in stage T4 (14.3% vs. 38.9%, P = 0.007) and N0 rate (34.6% vs. 88.9%, P < 0.001). Results indicated that ACC has a more local infiltration than NPC.
Imaging findings
All ACC lesions were located underneath submucosal space in pharyngonasal cavity without well-defined boundary. The nasopharyngeal mucosa space kept its integrity in 6 patients while the integrity of the mucosa was not maintained in 12 patients. Seven ACC patients showed masses with vast and obviously lobulated shapes spreading along interspaces around the cavity, whereas others showed incrassated mucosa with thickness of 5 mm to 9 mm. The sizes of these masses were 13 mm × 15 mm to 47 mm × 53 mm.
In the ACC group, lesions showed isosignal intensity on T1-weighted images (11 lesions with mixed hypointense, isointense, and hyperintense signals) and high signal intensity on enhanced T1-weighted images. Asymmetrical signal intensity mostly appeared in masses (e.g., four patients with high signal intensity, one with more or less high signal intensity, and two with isosignal intensity on T2-weighted images) (Figure 1A). Low signal intensity that appeared within lesions on enhanced T1-weighted images was different from those on T2-weighted images (Figure 2A). Results showed asymmetrical signal intensity in four patients with incrassated submucosal space in T2-weighted images and symmetrical signal intensity in seven patients. In the NPC group, most lesions showed low signal intensity on T1-weighted images, high signal intensity on T2-weighted images, and high signal intensity without well defined boundary on enhanced images (Figure 3).
Figure 1.
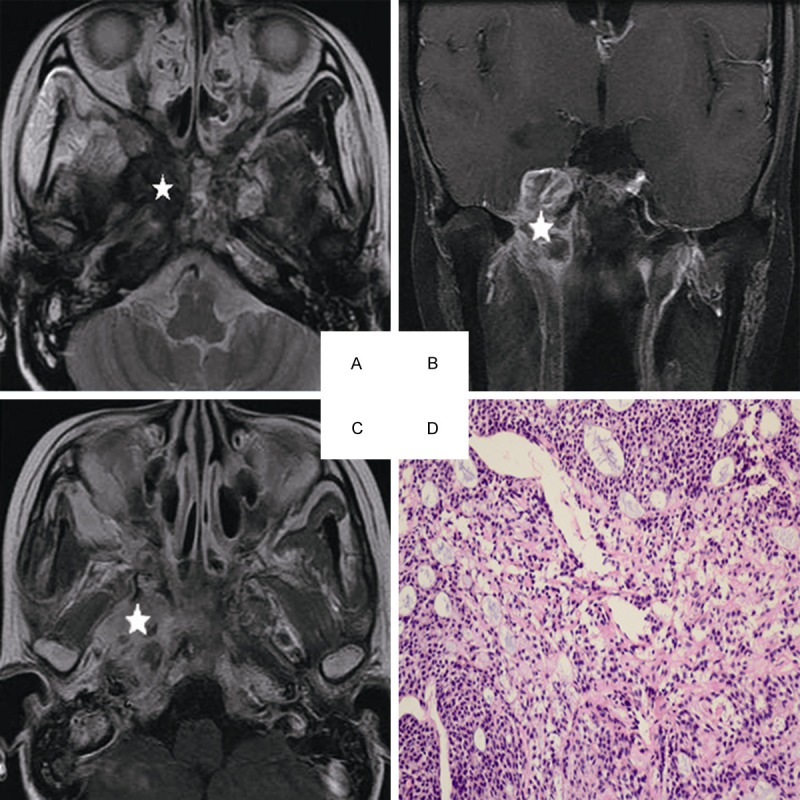
Head and neck MRI from a 25-year-old female with ACC of the nasopharynx. A. Axial plane, T2-weighted image. B. Coronal plane, T1-weighted image after contrast medium administration. C. Axial plane, T1-weighted image after contrast medium administration. D. The characteristic eosinophilic basement membrane material are displaying in pseudocysts. True gland lined by cuboidal epithelium are visible, (hematoxylin-eosin, original magnification × 200): The lesion invades to the pterygopalatine fossa, the parapharyngeal space, the pterygoid process and the skull base representing isosignal intensity on the T2-weighted image(white star), high signal intensity on the contrast-enhanced T1-weighted image (white star). There are mixed hypointense, isointense, and hyperintense in the lesion (white star).
Figure 2.
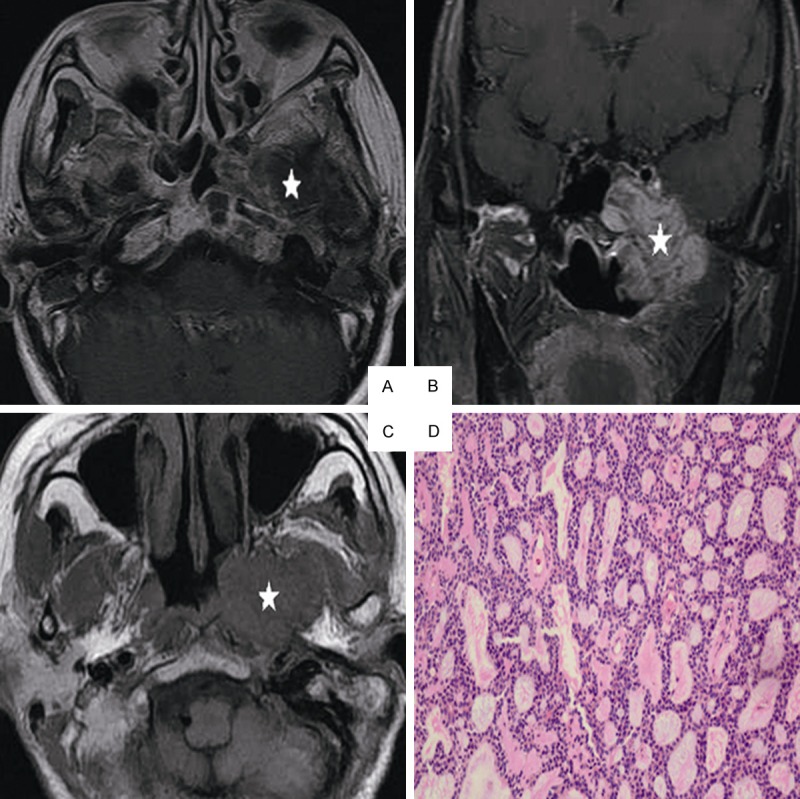
Head and neck MRI from a 51-year-old female with ACC of the nasopharynx. A. Axial plane, T1-weighted image after contrast medium administration. B. Coronal plane, T1-weighted image after contrast medium administration, C. Axial plane, T1-weighted image, D. Cribriform growth pattern displaying several prominent pseudocysts surrounded by basaloid cells (hematoxylin-eosin, original magnification × 200): The lesion invades the parapharyngeal space, the sphenoid sinus, the petrous apex and the muscles on the contrast-enhanced T1-weighted image without enhancement on the image (white star). On the T1-weighted image, the lesion represents low signal intensity invading the nasal cavity, the parapharyngeal space (white star).
Figure 3.
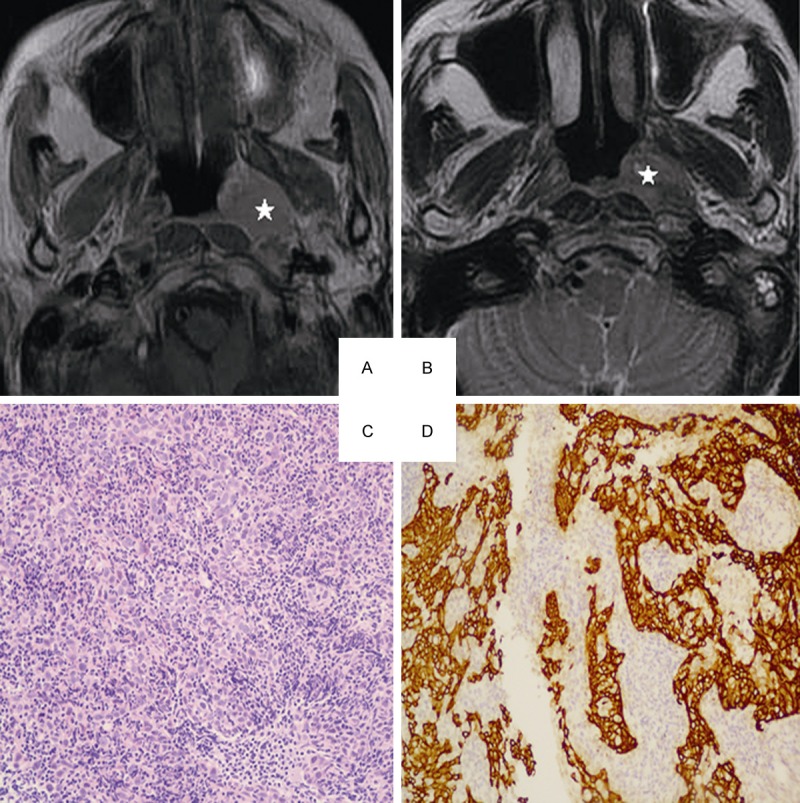
Head and neck MRI from a 53-year-old female with NPC. A. Axial plane, T1-weighted image after contrast medium administration, B. Axial plane, T1-weighted image after contrast medium administration: The lesion represents high signal intensity in part (white star). C. Well-formed syncytial nests of cells are separated by lymphoid cells. The nuclei are large and vesicular with prominent nucleoli (hematoxylin-eosin, original magnification × 200). D. The epithelial nature of the neoplasm is confirmed with keratin immunohistochemistry (CK 5/6, original magnification × 200).
In NPC patients, lymph nodes, parapharyngeal space, and skull base were commonly invaded with rates of 64.8%, 81.3%, and 53.3% respectively. The invasion rates of parapharyngeal space, skull base, and cavernous sinus were 77.8%, 66.7% and 33.3%, respectively, in ACC patients. The data were assessed by χ2 between the two groups. No statistical difference was observed in skull base invasion rate between ACC and common NPC (53.3% vs. 66.7%, P = 0.277), but the rate of local region invasion in ACC was significantly higher than common NPC (33.3% vs. 13.7%, P = 0.028). Conversely, the rate of lymph node metastases in NPC was higher than ACC (64.8% vs. 11.1%, P < 0.001). Results are displayed in Table 2.
Table 2.
MRI data in the two groups
| Local regions | NPC patients N (%) | ACC patients n (%) |
|---|---|---|
| Parapharyngeal space | 148 (81.3) | 14 (77.8) |
| Internal pterygoid muscle | 47 (25.8) | 6 (33.3) |
| External pterygoid muscle | 20 (11.0) | 5 (27.8) |
| Oropharynx | 15 (8.2) | 4 (22.2) |
| Nasal cavity | 53 (29.1) | 3 (16.7) |
| Skull base | 97 (53.3) | 12 (66.7) |
| Orbit | 13 (7.1) | 3 (16.7) |
| Infratemporal fossa | 9 (4.9) | 3 (16.7) |
| Accessory nasal sinuses | 43 (23.6) | 3 (16.7) |
| Laryngopharynx | 1 (0.5) | 3 (16.7) |
| Cavernous sinus | 25 (13.7) | 7 (33.3) |
| Cerebral parenchyma | 2 (1.1) | 1 (5.6) |
| Lymph node | 119 (64.8) | 2 (11.1) |
NPC: nasopharyngeal carcinoma; NACC: adenoid cystic carcinoma of the nasopharynx; n: number.
Seven patients showed 12 paralyzed cranial nerves (e.g., eight trigeminal nerves, two abducens nerves, one hypoglossal nerve, and one facial nerve) in the ACC group. Eleven patients showed 15 paralyzed cranial nerves (e.g., 10 trigeminal nerves, three abducens nerves, two hypoglossal nerves, and one oculomotor nerve) in the NPC group. The cranial nerve paralysis rate of ACC patients was higher than NPC patients (7 patients, 38.9% vs. 11, 6.0%, P < 0.001). Ten patients in the ACC group showed skull neuropore invasion and 59 patients in the NPC group showed neural foramen invasion, which indicates that the rate in ACC is higher than in NPC (55.6% vs. 32.4%, P = 0.049). The orbit and the medial segment of cranial nerves were high risk areas in ACC patients (33.3% vs. 13.2%, P = 0.022). Results are displayed in Table 3.
Table 3.
Relationship between appearance on MRI and cerebral nerveinvasion
| Cranial Nerve Lesions Location | NPC | ACC | ||
|---|---|---|---|---|
|
| ||||
| Patients n (%) | Paralytic cranial nerves (n) | Patients n (%) | Paralytic cranial nerves (n) | |
| Skull base or extracranial branch | ||||
| V3 extracranial branch | 53 (29.1) | V3 (9) | 12 (66.7) | V3 (8) |
| Pterygopalatina Fossa | 35 (19.2) | V2 (8) | 7 (38.9) | V2 (5) |
| Foramen lacerum | 17 (9.3) | V2 (7) | 3 (16.6) | V2 (3) |
| Foramen Ovale | 44 (24.1) | V3 (9) | 9 (50.0%) | V3 (8) |
| Jugular foramen | 9 (4.9%) | IX-XI (0) | 2 (11.1%) | IX-XI (0) |
| Hypoglossal canal | 21 (11.5%) | XII (2) | 5 (27.7%) | XII (1) |
| Internal acoustic meatus | 0 | 0 | 1 | VII (1) |
| Intracranial branch or orbit segment | ||||
| Superior orbital fissure | 3 (1.6%) | III (1) | 0 | 0 |
| V1 (3) | ||||
| Inferior orbital fissure | 12 (6.6%) | V2 (4) | 3 (16.6%) | V2 (1) |
| Cavernous sinus or Trigeminal ganglion | 25 (13.7%) | III (1) | 7 (38.9%) | V (8) |
| V (10) | VI (2) | |||
| VI (3) | ||||
V1 means the first branch of the nerve, V, III and VI mean the fifth, third and sixth cranial nerve.
Discussion
Epidemiology
NPC incidence is especially high in Guangdong province in southern China with obvious regionality. According to the global cancer statistics of 2008, the worldwide incidence and mortality were 0.2/100000 population and 0.1/100000 population, respectively [12]. The number in southern China is up to 30/100000 population to 80/100000 population, and males outnumber females by 2.3 to 1 [13]. NPC is known as a malignancy that is closely correlated with Epstein-Barr (EB) virus infection. EB virus infection rate in NPC is estimated at almost 90% [14]. Viral capsule antigen/IgA examination is sensitive to EB virus infection which assists in NPC diagnosis. Even in southern China, a high risk area for NPC, ACC of the nasopharynx is still rare. The correlation between ACC and EB virus infection is still unclear. Female patients outnumber males. No evidence showing that ACC correlates with EB virus infection exists. The difference in incidence between ACC and NPC indicates different disease causes.
Clinical symptoms
The most common symptoms include resorptive nosebleeds, nasal blockage, and masses in neck that appear much earlier in NPC patients resulting in earlier diagnosis [15]. These symptoms are also common in ACC patients but appear later than NPC because of slow disease progression. Cranial nerves paralysis and stage T4 are more frequently diagnosed in ACC patients [16]. ACC prefers local infiltration to other adjacent tissues whereas NPC tends to present as lymph node metastasis.
Extension patterns
Two common patterns for extension in carcinomas of the nasopharynx exist [17]. One is aggressive local infiltration, whereas the other is spreading along well-defined routes, such as neural canal or lymph vessels [18]. Local infiltration indicates growth of masses and invasion of adjacent skull base. Another characteristic is coarse cranial nerves or extended cranial nerve canal or lymph node metastases. Aggressive local infiltration and perineural infiltration usually occur in the ACC of the nasopharynx, whereas lymph node metastasis frequently occurs in the NPC.
MRI displays invasion of cranial nerves with good resolution, which results in better diagnosis than clinical examination in medical practice. Little resistance to tumor invasion is observed in cranial nerves causing lack of typical neural symptoms and negative examinations in the early stage of the disease [3,8]. In the present study, ACC of the nasopharynx is characterized by more frequent spreading along the nerve canal. Trigeminal nerve invasion is most frequently detected in ACC patients. A well-defined extension route for ACC is demonstrated: pharyngonasalcavity→parapharyngealspace→trigeminalnerve→foramenovale→cavernous sinus. The cavernous sinus is more easily involved in ACC patients because III-VI nerves pass through it (Figure 1B and 1C). The trigeminal nerve, the abducens nerve, and the oculomotor nerve are more frequently involved (Figure 2B and 2C). Invasion to inferior orbital fissure is also through the following route: retropharyngeal space→hypoglossalcanal→pterygopalatine fossa or cranial fossa.
Abundant lymph vessels underneath the nasopharyngeal mucosa space result in higher frequency of lymph node metastasis diagnosis in NPC patients. The patients come to the hospital primarily for masses in the neck. In a survey of 271 new cases marked as NPC, 204 patients (75.3%) had lymph node metastases [19]. Another study showed that the percentage increased to 80% (91/114 patients) during MRI examinations [20]. Lymph node metastases rate reaches 11.1% in ACC patients and 64.8% in NPC patients. NPC prefers extension through lymph vessels, whereas this tendency is not present in ACC.
Treatments
Concurrent chemoradiotherapy is the standard modality for NPC, but there is no confirmed evidence for its application in ACC therapy. Therapeutic strategy depends on the pathophysiological characters of ACC, which tends to show local infiltration and neural extension. Many other series of ACC have been described. A retrospective study suggested that surgery combined with radiotherapy should be performed on these patients. Nevertheless, postoperative radiotherapy did not result in a significant effect according to other studies. ACC of the nasopharynx is characterized by high incidence of PNI, frequently aggressive local infiltration, and infrequently, lymph node metastasis, which indicates that radiotherapy is the first choice for treatment. Intensity modulated radiation therapy should be performed on extended planning target volume. No evidence exists that chemotherapy would improve the prognosis of ACC patients after radiotherapy.
Conclusion
The present study demonstrates that ACC is a malignancy of the nasopharynx characterized by local infiltration and neural invasion. ACC tends to involve aggressive local infiltration and extension along the cranial nerve canal. The appearance forms special characters on MRI images of ACC of the nasopharynx, which helps with more accurate MRI diagnosis.
Acknowledgements
We give thanks to the department of the radiology, cancer center, Sun Yet-San University.
Disclosure of conflict of interest
None.
References
- 1.Tsui EY, Chan JH, Ramsey RG, Leung TW, Cheung YK, Luk SH, Lai KF, Wong KP, Fong D, Yuen MK. Late temporal lobe necrosis in patients with nasopharyngeal carcinoma: evaluation with combined multi-section diffusion weighted and perfusion weighted MR imaging. Eur J Radiol. 2001;39:133–8. doi: 10.1016/s0720-048x(01)00328-x. [DOI] [PubMed] [Google Scholar]
- 2.JH , PZ , WQ C, editors. Annual Report of Tumor in China. Beijing: Military Medical Science Publishing Company; 2013. [Google Scholar]
- 3.Barrett AW, Speight PM. Perineural invasion in adenoid cystic carcinoma of the salivary glands: A valid prognostic indicator? Oral Oncology. 2009;45:936–40. doi: 10.1016/j.oraloncology.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Kokemueller H, Eckardt A, Brachvogel P, Hausamen JE. Adenoid cystic carcinoma of the head and neck-a 20 years experience. Int J Oral Maxillofac Surg. 2004;33:25–31. doi: 10.1054/ijom.2003.0448. [DOI] [PubMed] [Google Scholar]
- 5.Zhang WD, Li CX, Liu QY, Hu YY, Cao Y, Huang JH. CT, MRI, and FDG-PET/CT imaging findings of abdominopelvicdesmoplastic small round cell tumors: correlation with histopathologic findings. Eur J Radiol. 2011;80:269–73. doi: 10.1016/j.ejrad.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 6.Huang B, Wong CS, Whitcher B, Kwong DL, Lai V, Chan Q, Khong PL. Dynamic contrast-enhanced magnetic resonance imaging for characterising nasopharyngeal carcinoma: comparison of semiquantitative and quantitative parameters and correlation with tumour stage. Eur Radiol. 2013;23:1495–502. doi: 10.1007/s00330-012-2740-7. [DOI] [PubMed] [Google Scholar]
- 7.Sharma M, Bartlett E, Yu E. Metastatic retropharyngeal lymph nodes in nasopharyngeal carcinoma: imaging criteria. Expert Rev Anticancer Ther. 2010;10:1703–6. doi: 10.1586/era.10.159. [DOI] [PubMed] [Google Scholar]
- 8.Dubrulle F, Souillard R, Hermans R. Extension patterns of nasopharyngeal carcinoma. Eur Radiol. 2007;17:2622–30. doi: 10.1007/s00330-007-0616-z. [DOI] [PubMed] [Google Scholar]
- 9.Liu L, Liang S, Li L, Mao Y, Tang L, Tian L, Liao X, Cui C, Lin A, Ma J. Prognostic impact of magnetic resonance imaging-detected cranial nerve involvement in nasopharyngeal carcinoma. Cancer. 2009;115:1995–2003. doi: 10.1002/cncr.24201. [DOI] [PubMed] [Google Scholar]
- 10.Pan J, Xu Y, Qiu S, Zong J, Guo Q, Zhang Y, Lin S, Lu JJ. A Comparison between the Chinese 2008 and the 7th edition AJCC staging systems for nasopharyngeal Carcinoma. Am J Clin Oncol. 2013;38:189–96. doi: 10.1097/COC.0b013e31828f5c96. [DOI] [PubMed] [Google Scholar]
- 11.Luo S, Zhao L, Wang J, Xu M, Li J, Zhou B, Xiao F, Long X, Shi M. Clinical outcome for earlystage nasopharyngeal carcinoma with predominantly WHO II histology treated by intensity-modulated radiation therapy with or without chemotherapy in non-endemic region of China. Head Neck. 2014;36:841–7. doi: 10.1002/hed.23386. [DOI] [PubMed] [Google Scholar]
- 12.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 13.Li GL, Chen WQ. Representativeness of population-based cancer registration in China--comparison of urban and rural areas. Asian Pac J Cancer Prev. 2009;10:559–64. [PubMed] [Google Scholar]
- 14.Dogan S, Hedberg ML, Ferris RL, Rath TJ, Assaad AM, Chiosea SI. Human papillomavirus and Epstein-Barr virus in nasopharyngeal carcinoma in a low-incidence population. Head Neck. 2014;36:511–6. doi: 10.1002/hed.23318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balachandran R, Philip R, Avatar S, Simon R, Mann GS, Benedict CT, Amy CA, Ch’ng ML. Exploring the knowledge of nasopharyngeal carcinoma among medical doctors at primary health care level in Perak state, Malaysia. Eur Arch Otorhinolaryngol. 2012;269:649–58. doi: 10.1007/s00405-011-1665-0. [DOI] [PubMed] [Google Scholar]
- 16.Liu TR, Yang AK, Guo X, Li QL, Song M, He JH, Wang YH, Guo ZM, Zhang Q, Chen WQ, Chen FJ. Adenoid cystic carcinoma of the nasopharynx: 27-year experience. Laryngoscope. 2008;118:1981–8. doi: 10.1097/MLG.0b013e3181801d23. [DOI] [PubMed] [Google Scholar]
- 17.Mo L, Weng J, Zeng F, Li X, Liu B, Li Z, Kuang G. [The relationship between extend types and distant metastasis of nasopharyngeal carcinoma] . Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010;24:554–5. 558. [PubMed] [Google Scholar]
- 18.Li WF, Sun Y, Chen M, Tang LL, Liu LZ, Mao YP, Chen L, Zhou GQ, Li L, Ma J. Locoregional extension patterns of nasopharyngeal carcinoma and suggestions for clinical target volume delineation. Chin J Cancer. 2012;31:579–87. doi: 10.5732/cjc.012.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su Y, Zhao C, Li WJ, Deng XY, Zeng RF, Cui NJ, Lu TX. CT-guided needle biopsy through mandibular area for the diagnosis of nasopharyngeal carcinoma in the parapharyngeal space. Chin J Cancer. 2010;29:768–73. doi: 10.5732/cjc.009.10782. [DOI] [PubMed] [Google Scholar]
- 20.Zhang YL, Li J, Mo HY, Qiu F, Zheng LM, Qian CN, Zeng YX. Different subsets of tumor infiltrating lymphocytes correlate with NPC progression in different ways. Mol Cancer. 2010;9:4. doi: 10.1186/1476-4598-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


