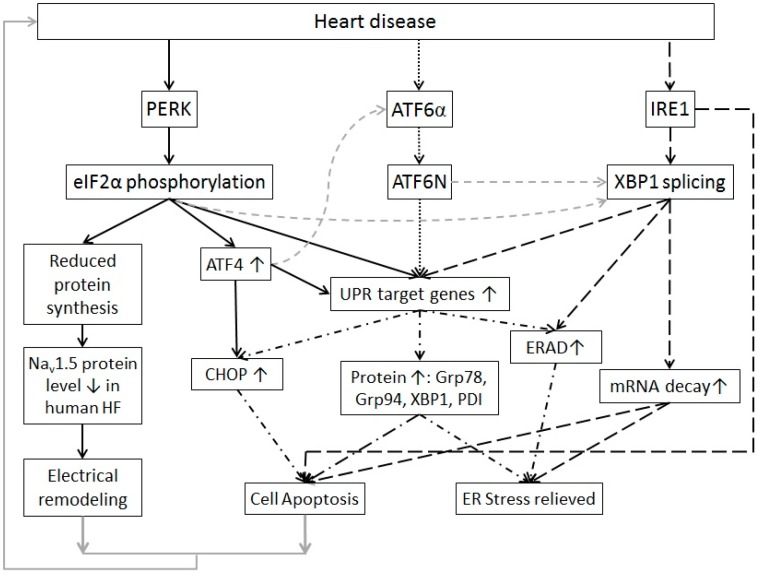
Figure 1.
A scheme of signaling pathways of the unfolded protein response with its sensors, downstream effectors, targeting genes, and possible outcomes. The PERK branch (linked by black-solid arrows) can be activated in human heart failure, diabetes hearts and ischemia/reperfusion, leading to inhibition of nascent protein synthesis and ATF4 elevation [10,11,12,13]. Elevated ATF4 can induce expression of CHOP and other UPR target genes. The ATF6 branch (linked by black-dotted arrows) is reported to be activated in early stage of ischemia and mainly induces UPR target genes expression [14,15,16]. The IRE1 branch (linked by black-dashed arrows) is reported to be activated in human heart failure and ischemia/reperfusion [12,17,18]. Its downstream effectors activate the ERAD and ER-localized mRNA decay [5]. The IRE1 branch has also been reported to promote cell apoptosis via Jun N-terminal kinase and p38 MAPK [19]. All three branches cause UPR target genes expression (linked by dash dotted dash arrows) such as the UPR chaperones Grp78 and Grp94 and ERAD proteins to help misfolded/unfolded protein refold or degrade. There is also crosstalk among the three branches (linked by gray dashed arrows). Under short-term and mild ER stress, the UPR is adaptive to release the ER stress; while under prolonged and severe ER stress, the UPR leads to cell apoptosis and electrical remodeling (due to decreased cardiac ion channels) that will harm the cardiac function and lead to heart diseases (linked by gray arrows).