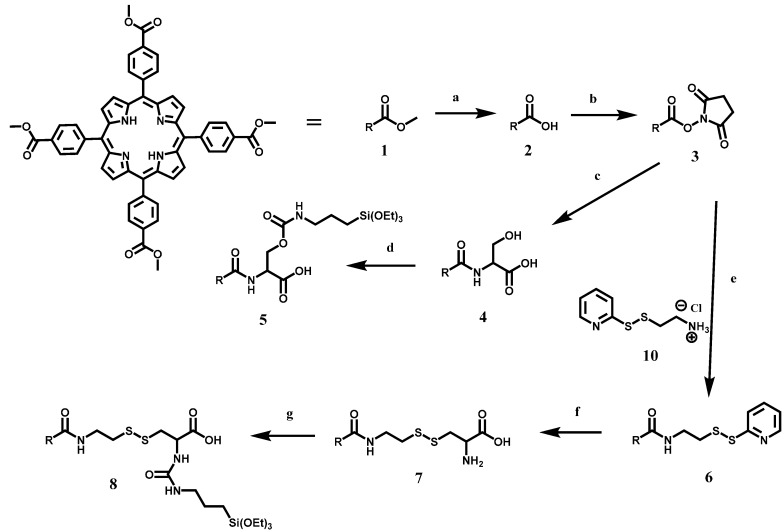
Scheme 3.
Schematic representation of the synthesis of C-TCPP and RR-TCPP silane derivatives. For simplification, the tetrakis(phenyl) porphyrin is represented as the R group. See details of the experimental conditions for each of the reactions in the Experimental Section. The synthesis of 5,10,15,20-tetrakis(4-carbomethoxyphenyl) porphyrin (TCM4PP) (1) is carried out by the condensation of pyrrole and benzaldehyde; (a) Hydrolysis of 1 under basic conditions afforded TCPP (2); (b) Compound 2 can be activated toward acyl nucleophilic substitution by the formation of the ester bond with N-hydroxysuccinimide (NHS) (3); (c) For the synthesis of C-TCPP, compound 3 underwent an acyl nucleophilic substitution with serine to produce TCPP-Serine (4); (d) Finally, 4 reacted with triethoxysilane propyl isocyanate (TES-PI) to obtain C-TCPP (5); (e) To produce RR-TCPP, compound 3 also underwent acyl nucleophilic substitution with pyridyl disulfide cysteamine (PDSCA) (10) to form TCPP-PDSCA (6); (f) Compound 7 is synthesized by the disulfide exchange reaction between 6 and cysteine; (g) Lastly, RR-TCPP (8) is produced by reacting 7 with TES-PI.