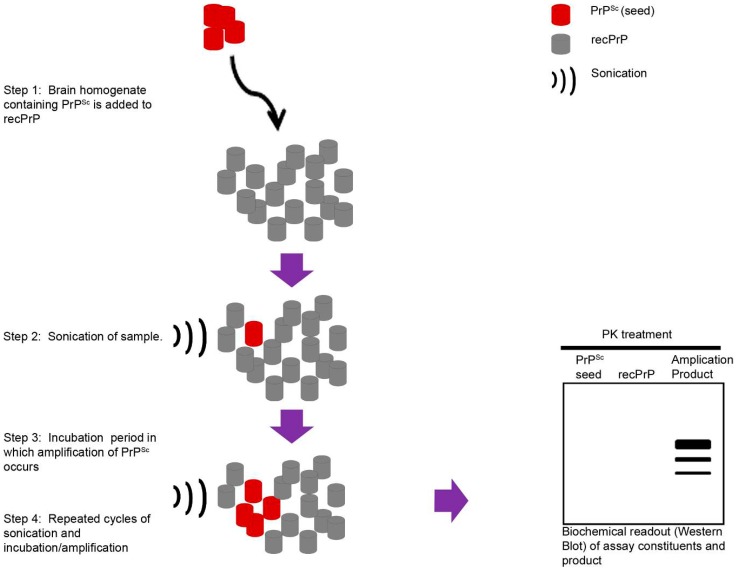
Figure 1.
Diagrammatic representation of cell-free conversion assays. A small quantity of a brain homogenate containing PrPSc, usually at quantities lower than is normally detectable when immunoblotting, is added to a pool of “normally” folded host-encoded protein. This can be either purified recombinant proteins (recPrP) or an uninfected brain homogenate. Briefly, the assays undergo periods of incubation, whereby the PrPSc seeds can interact with recPrP and cause protein misfolding and aggregation. Intermittently, periods of sonication are used to break down larger aggregates to allow further conversion of recPrP to PrPSc. The assay products are then assessed using Western blot analysis. These systems result in a large accumulation of PrPSc, which have occurred in a cell-free system, and, as the result of protein-templated conversion directed from the initial extremely small quantity of PrPSc added to the reaction in the first instance.