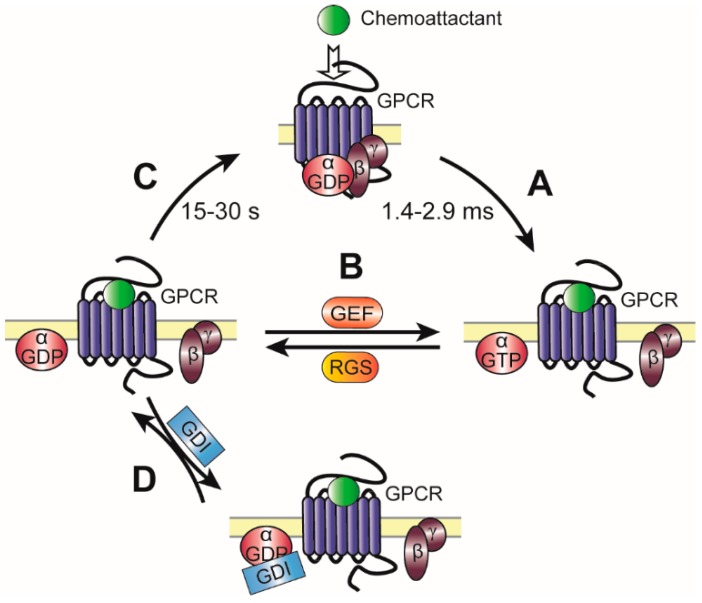
Figure 2.
A schematic representation of mammalian Gα regulation. Upon binding of extracellular chemoattractant, GPCRs undergo conformational changes to act as guanine nucleotide exchange factors (GEFs) for Gα subunits, facilitating GDP release and subsequent binding of GTP, and release from Gβγ dimers (A) Non-receptor GEFs can bind to Gα-GDP and extend Gα subunit activation by stimulating the exchange of Gα-GDP to the active GTP-bound state. Regulator of G protein signaling (RGS) proteins stimulate the exchange of Gα-GTP back to Gα-GDP, serving as GTPase-accelerating proteins (GAPs) for Gα, thereby dramatically enhancing their intrinsic rate of GTP hydrolysis; (B) Upon GTP hydrolysis of Gα, the heterotrimer of Gα-GDP and Gβγ can reform, restoring the coupled GPCR/G protein complex; (C) However, in the presence of guanine nucleotide dissociation inhibitors (GDIs), Gα can become trapped in a Gα·GDP/GDI complex, preventing Gβγ from reassociation and re-coupling to GPCRs (D).