Fig. 4. Intermediates of SR1 protein-folding cycle.
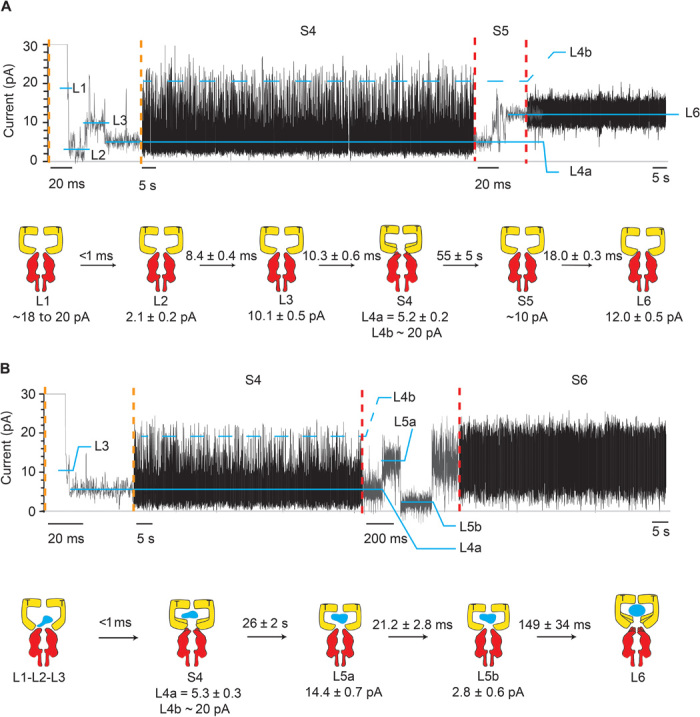
Typical SR1 current blockades. The current traces between the vertical dotted orange and red lines are expansions of the main current traces showing the fast current transitions that characterize the L1, L2, L3, and S5 states. The cartoon depictions below the current traces show the kinetic succession between SR1 intermediates. SR1 is in yellow, αHL-GroESL is in red, and the substrate protein is in blue. (A) αHL-GroESL current blockade for the ternary SR1:ATP:αHL-GroESL complex. L1 (dwell time, <1 ms) was observed only in a few blockades (6%). Ninety-two percent of blockades started with either L2 (77%) or L3 (15%) current levels. The transition from S4 to L6 was fast (10.3 ± 0.6 ms; fig. S5) and characterized by current fluctuations showing an average value at ~10 pA. An expansion of the S4 current state is shown in fig. S5. (B) αHL-GroESL current blockade for the quaternary SR1:ATP:DHFR:αHL-GroESL complex. In 91% of blockades, L1, L2, and L3 current levels could not be sampled, whereas the transition between S4 and L6 showed two well-defined current levels, indicating that the unfolded protein modified the interaction of SR1 with αHL-GroESL (see the text). Dihydrofolate reductase (DHFR) was urea unfolded and incubated with SR1 before addition to the cis chamber. Traces were recorded in 1 M KCl and 50 mM tris-HCl (pH 7.5) at 23°C in the presence of 1 mM ATP and 5 mM MgCl2 by applying a 10-kHz low-pass Bessel filter and using a 20-μs (50-kHz) sampling rate. An additional postacquisition Gaussian filter at 2 kHz was then applied to the current traces.
