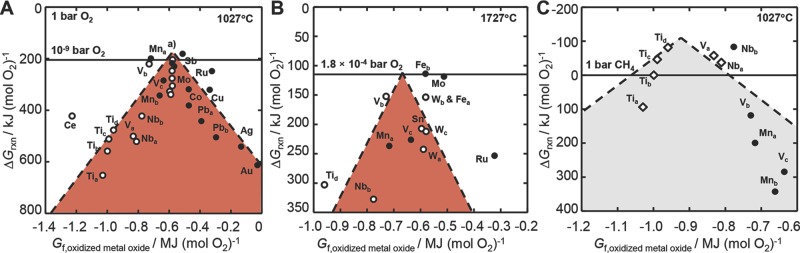
Figure 1.
Volcano-type plots for isothermal redox cycles with solid metal oxides. A) The limiting free energy of oxidizing a metal oxide with CO2 (filled circles, Equation 1 and reducing a metal oxide in an inert atmosphere (empty circles, Equation 3 is plotted versus the formation energy of the oxidized metal oxide. The analysis indicates increased reaction yields (less endergonic redox cycles at the top of the volcano) when B) increasing the temperature and decreasing the partial O2 pressure or C) coupling the metal oxide reduction with reforming of methane (empty diamonds, Equation 4. The metal oxide pairs are (Tia) TiO/Ti2O3, (Tib) Ti2O3/Ti3O5, (Tic) Ti3O5/Ti4O7, (Tid) Ti4O7/r-TiO2, (Va) VO/V2O3, (Vb) V2O3/V2O4, (Vc) V2O4/V2O5, (Mna) MnO/Mn3O4, (Mnb) Mn3O4/Mn2O3, (Fea) FeO/Fe0.947O, (Feb) FeO/Fe3O4, (Fc) Fe3O4/Fe2O3, (Co) CoO/Co3O4, (Cu) Cu2O/CuO, (Nba) NbO/NbO2, (Nbb) NbO2/Nb2O5, (Mo) MoO2/MoO3, (Ru) Ru/RuO2, (Ag) Ag/Ag2O, (Sn) SnO/SnO2, (Sb) c-Sb2O3/SbO2, (Ce) Ce2O3/CeO2, (Wa) WO2/WO2.72, (Wb) WO2.72/WO2.9, (Wc) WO2.9/WO2.96, (Wd) WO2.96/WO3, (Au) Au/Au2O3, (Pba) PbO/Pb3O4, (Pbb) Pb3O4/PbO2. a) Starting with the most endergonic cycle, Wa, Sn, Wc, Feb, Wb, Fec, Fea, and Wd. Dashed lines are linear fits.