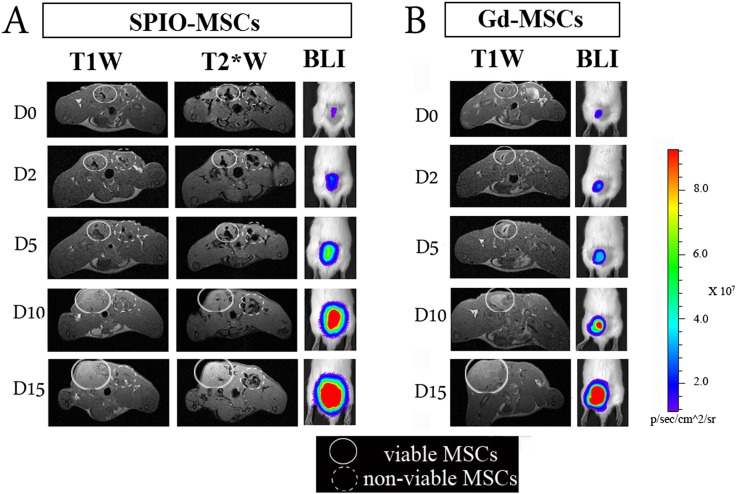
Figure 3.
Monitoring the cellular status of cells by situation-dependent contrast behaviour of Gd. MSCs were labelled with either Gd–liposomes or iron oxide particles and an optical reporter gene (firefly luciferase). Following labelling, cell populations were split in two identical samples. One part was then submitted to repeated freeze-thawing to generate non-viable intact cells. Dual-labelled cells were injected intramuscularly into the lower back of rats, i.e. viable labelled cells on the left side and non-viable cells on the right side. Rats were imaged by MRI (3.0 T) and bioluminescent imaging at several time points over a period of 2 weeks. (a) SPIO–MSCs caused a signal void (hypointensity), regardless of the cell viability. In the acute post-transplantation stage, no substantial differences in visual appearance were detected between viable and non-viable SPIO-MSCs. (b) Viable Gd–MSCs showed a different dynamic signal behaviour compared with non-viable MSCs. Immediately post-transplantation, viable MSCs were consistently detected as a hypointense area on T1 weighted scans (“quenched signal intensity”), whereas a similar density of non-viable Gd–MSCs resulted in increased signal intensity (hyperintensity) at the injection site. In contrast to SPIO–MSCs, hyperintense signal from non-viable Gd–MSCs had already resolved after 2 h post-transplantation. An increased signal intensity on BLI images reflects the cell proliferation that contributed to the tracer dilution observed by MRI.T1W, T1 weighted; T2*W, T2* weighted; BLI, bioluminescence imaging; MSCs, mesenchymal stem cells. Reprinted from Guenoun et al71 with permission from John Wiley and Sons.