Figure 3. Expressing receiver proteins at optimal levels minimizes crosstalk while maintaining sensitivity in dual‐channel reporter constructs.
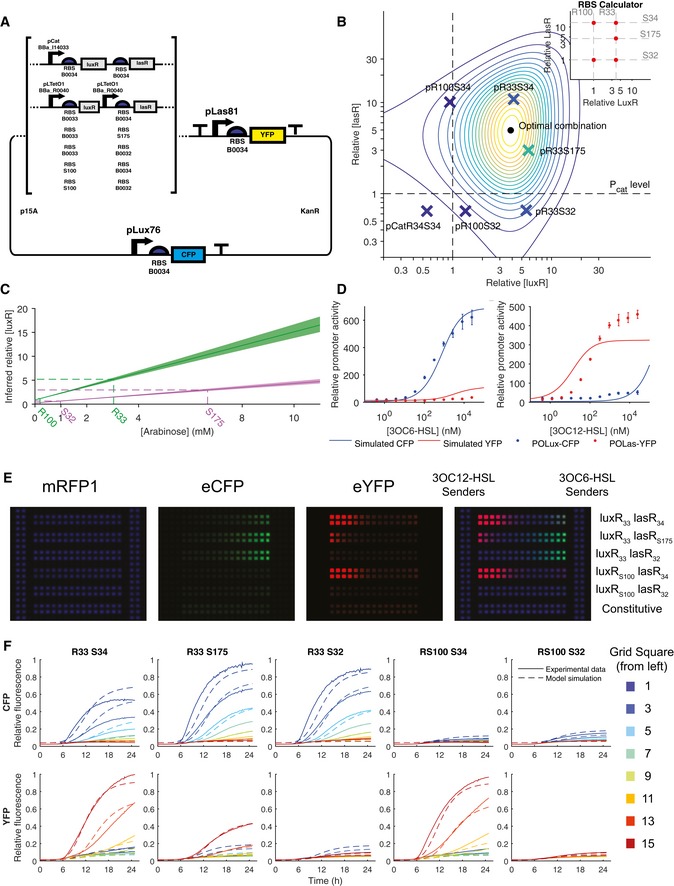
- Double reporter constructs express eYFP under the control of pLas81 and eCFP under pLux76. Receiver proteins are expressed under the control of pCat (Bba_I14033), pLlacO1 (Bba_R0011), or pLTetO1 (Bba_R0040) and also vary in the RBS used to control translation.
- A fully parameterized model is used to predict the optimal expression levels for LuxR and LasR. The point at which expression levels of LuxR and LasR result in the maximal simulated signal to crosstalk ratio is labeled with a dot while isolines are colored to represent lower values of that ratio. Double reporters expressing eCFP under the control of pLux76 and eYFP under the control of pLas81 along with both receiver proteins under the control of various promoters and RBS sequences (see Appendix Table S2) are each represented with an “X” placed at the expression levels of LuxR and LasR that were inferred from measuring the plasmid's response to 3OC6HSL and 3OC12HSL in both the eCFP and eYFP channels (ratiometrically with respect to signal from a chromosomally integrated constitutively expressed mRFP1). The color of the X reflects the actual ratio of signal to crosstalk of the data for that receiver, on the same scale as the isolines. Expression levels of LuxR and LasR are normalized to the levels of expression in the ratiometric reporters driven by pCat.
- Constitutive receiver protein concentration in double reporters is equivalent to that of inducible expression at interpolated arabinose concentrations. Shown are the best‐fit relationships between LuxR (green) or LasR (pink) concentration and arabinose for all experiments involving pBAD‐LuxR and pBAD‐LasR (lines), with standard deviations computed from 5,000 MCMC samples (shading). Also indicated are the relative LuxR/LasR concentrations inferred for double reporter constructs in pCat units.
- Activity (relative to chromosomal constitutive mRFP1) of pLux76 (eCFP, blue) and pLas81 (eYFP, red) in the pR33S175 construct as a function of 3OC6HSL (top) or 3OC12HSL (bottom). Points indicate the mean of three replicates and error bars indicate the standard deviation while lines indicate the mean of the best‐fit models. Simulations used LuxR and LasR levels indicated in (A) (r = 5.89, s = 2.97), and all other parameters as specified in Appendix Table S6.
- Image at t = 1,500 min of chromosomal constitutive mRFP1 cells containing each of the double reporters or a control construct constitutively expressing eCFP and eYFP (pPRYFPPRCFP), plated on a membrane printed with a hydrophobic grid along with 3OC6HSL sender cells and 3OC12HSL sender cells.
- Activity (relative to chromosomal constitutive mRFP1) of pLux76 (eCFP, top) and pLas81 (eYFP, bottom) for each double reporter is plotted against time for every other grid square according to the color scheme shown. Experimental data are plotted as a solid line while model simulation is plotted as a dotted line.
Source data are available online for this figure.
