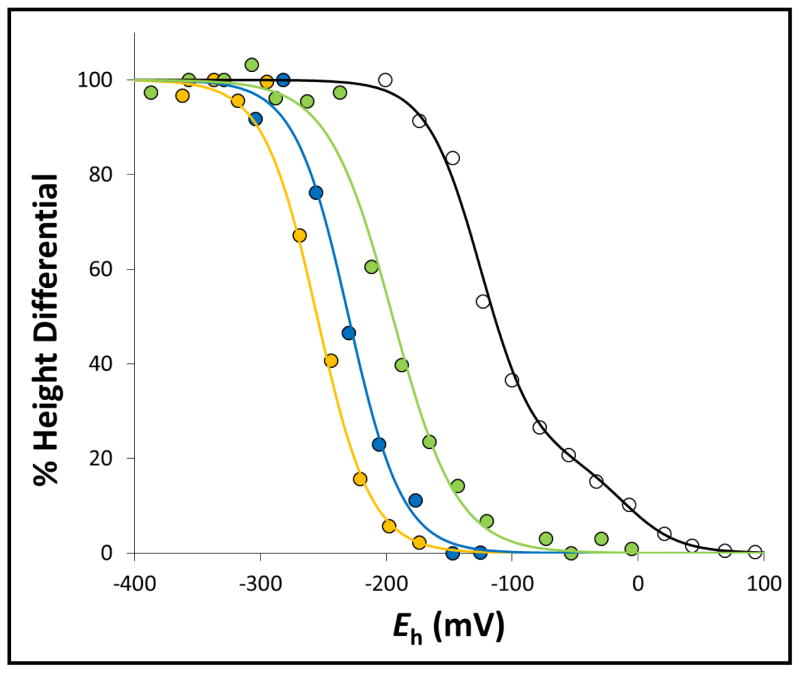
Figure 6. Redox-dependent interplay between the g =1.91 and g = 1.92 components of the [2Fe-2S] cluster EPR spectrum.
The height differentials between the g = 1.91 and g = 1.92 components of the [2Fe-2S] cluster EPR signal as a function of ambient potential were plotted for wild-type SdhCDAB (black), SdhA-H45A (green), SdhA-R286A (yellow), and SdhA-R286K (blue) and fitted to the Nernst equation. For titration of the wild-type SdhCDAB enzyme, a second component corresponding to the reduction of the [2Fe-2S] cluster itself was also modeled.