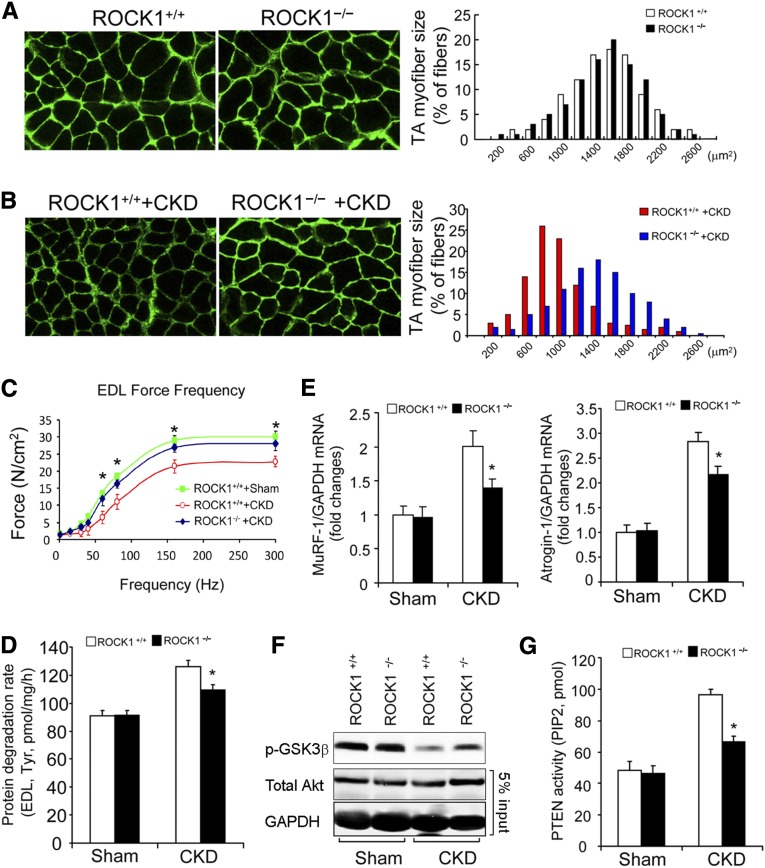
Figure 7.
ROCK1 null suppresses CKD-induced muscle proteolysis. (A) In the TA muscles of sham-operated, ROCK+/+ and ROCK1 KO mice, the myofibers sizes were assessed as a distribution of the myofibers’ CSA (n=3, approximately 300 myofibers were measured in each mouse). (B) Leftward-shift of distribution of the myofibers’ CSA in TA muscles of ROCK+/+ mice with CKD was prevented in ROCK1−/− mice with CKD (n=5, approximately 300 myofibers in muscle of each mouse were examined). (C) CKD impairs the contractile function of EDL muscles of ROCK+/+ mice. The decreased contractile force caused by CKD was improved in ROCK1−/− mice despite CKD (mean±SEM, *P<0.05, ROCK1−/− +CKD versus ROCK1+/+ +CKD; n=3–5 in each group). (D) Rates of protein degradation, assessed by tyrosine release isolated EDL muscle, were significantly suppressed in ROCK1 KO mice with CKD (mean±SEM; n=5–7 in each group; *P<0.05 versus ROCK+/++CKD). (E) The expressions of Atrogin-1/MAFbx and MuRF-1 (quantitative RT-PCR) were significantly suppressed in muscles of ROCK1−/− mice with CKD (mean±SEM, *P<0.01 versus ROCK+/+with CKD, n=5). (F) Phosphorylation of Akt was examined by western blotting. CKD suppressed Akt activity in muscles of ROCK+/+ mice. This response was blunted in muscles of ROCK1−/− mice with CKD. (G) PTEN activities in the absences or presence of CKD were examined in gastrocnemius muscles of ROCK+/+ and ROCK1−/− mice. The absence of ROCK1 reduced PTEN activity stimulated by CKD (mean±SEM; *P<0.01 versus ROCK+/+ mice with CKD; n=5).