Abstract
CKD leads to disturbances in multiple interrelated hormones that regulate bone and mineral metabolism. The renal handling of mineral metabolism hormones in humans is incompletely understood. We determined the single-pass renal clearance of parathyroid hormone, fibroblast growth factor 23, vitamin D metabolites, and phosphate from paired blood samples collected from the abdominal aorta and renal vein in 17 participants undergoing simultaneous right and left heart catheterization and estimated associations of eGFR with the renal elimination of metabolites. The mean age ±SD of the study population was 71.4±10.0 years and 11 participants (65%) were male. We found a relatively large mean±SD single-pass renal extraction of parathyroid hormone (44.2%±10.3%) that exceeded the extraction of creatinine (22.1%±7.9%). The proportionate renal extraction of parathyroid hormone correlated with eGFR. The renal extraction of fibroblast growth factor 23 was, on average, lower than that of parathyroid hormone with greater variability across individuals (17.1%±19.5%). There were no differences in the mean concentrations of vitamin D metabolites across the renal vein and artery. In summary, we demonstrate substantial single-pass renal extraction of parathyroid hormone at a rate that exceeds glomerular filtration. Impaired renal clearance of parathyroid hormone may contribute to secondary hyperparathyroidism in CKD.
Keywords: renal clearance, extraction, mineral metabolism biomarkers
CKD leads to disturbances in multiple interrelated hormones that regulate bone and mineral metabolism.1 Serum concentrations of parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF23) rise early during the course of CKD in parallel with a decline in 1,25-dihydroxyvitamin D (1,25[OH]2D) and 24,25-dihydroxyvitamin D (24,25[OH]2D).2,3 Known causes of these hormonal changes include alterations in circulating calcium and phosphate concentrations and inadequate production of 1,25(OH)2D and klotho by the failing kidneys.
The renal handling of mineral metabolism hormones in humans is incompletely understood. Older studies using early PTH antibodies suggested renal clearance of PTH via peritubular uptake and, to a lesser extent, glomerular filtration. Some experimental studies also indicate that impaired degradation of PTH contributes to the high circulating levels of PTH in CKD.4–6 FGF23 is produced in bone and cleaved in the circulation; however, little is known about the renal clearance of FGF23. It is possible that reduced renal clearance of mineral metabolism hormones contributes to their accumulation in CKD.
To understand how the human kidney modulates circulating biomarkers of mineral metabolism, we determined the single-pass renal clearance of PTH, FGF23, vitamin D metabolites, and phosphate from paired blood samples collected from the abdominal aorta and renal vein among patients undergoing left and right heart catheterization.
Results
The mean age of the study population was 71±10 years; 11 participants (65%) were male. There were seven participants who were using vitamin D supplements; none of the study participants used calcitriol (Table 1). The mean eGFR was 66 ml/min per 1.73 m2 (interquartile range [IQR], 55–78 ml/min per 1.73 m2). Mean (±SD) values of PTH, FGF23, and 1,25(OH)2D3 in the renal artery were: 62.9±31.1 pg/ml, 73.2±36.1 pg/ml, and 27.7±14.0 pg/ml, respectively.
Table 1.
Characteristics of individuals undergoing right and left heart catheterization
| Characteristics | |
|---|---|
| N | 17 |
| Age (years) | 71.4±10.0 |
| Male | 11 (65%) |
| Body mass index (kg/m2) | 27.3±6.5 |
| eGFR (ml/min per 1.73 m2) | 65.9±18.2 |
| Hypertension | 16 (94%) |
| Type 2 diabetes | 4 (24%) |
| Heart failure | 6 (35%) |
| ACE inhibitors/ARB use | 12 (71%) |
| Statin use | 12 (71%) |
| Vitamin D supplement use | 6 (35%) |
Values are mean±SD or frequency and percentage.
ACE, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; eGFR, estimated glomerular filtration rate based on CKD-EPI 2009 (creatinine).
The single-pass renal elimination of creatinine was 22.1±7.9% (Table 2). The mean concentrations of PTH, FGF23, and phosphate were significantly lower in the renal vein compared with the renal artery. The mean concentration of 1,25(OH2)D2 and other vitamin D metabolites were numerically and statistically similar in the renal vein compared with the artery with a wide range of extraction values among the study population.
Table 2.
Renal arterial and venous plasma concentrations among 17 subjects undergoing heart catheterization
| Artery (Mean±SD) | Vein (Mean±SD) | P Valuea | Single-Pass Renal Elimination (%) | Urine (n=7)(Mean±SD) | ||
|---|---|---|---|---|---|---|
| Mean±SD | IQR | |||||
| Creatinine (mg/dl) | 1.10±0.4 | 0.9±0.3 | <0.001 | 22.1±7.9 | 16.8, 25.9 | 91.6±46.7 |
| Mineral metabolism markers | ||||||
| Parathyroid hormone (pg/ml) | 62.9±31.1 | 35.9±20.6 | <0.001 | 44.2±10.3 | 38.0, 50.7 | 0 |
| Fibroblast growth factor-23 (pg/mL) | 73.2±36.1 | 61.5±37.7 | 0.01 | 17.1±19.5 | 0.9, 30.0 | 15.9±15.2 |
| Phosphate (mg/dl) | 3.7±0.7 | 3.5±0.6 | 0.03 | 5.5±9.4 | 0.0, 12.8 | 45.5±26.2 |
| 1,25-dihydroxyvitamin D3 (pg/ml) | 27.7±14.0 | 27.4±14.2 | 0.85 | 1.4±22.4 | −7.7, +15.2 | 0 |
| 24,25-dihydroxyvitamin D3 (ng/ml) | 3.3±2.1 | 3.3±2.1 | 0.78 | −2.1±21.5 | −5.4, +8.5 | 0 |
| 25-hydroxyvitamin D2 (ng/ml) | 3.5±5.1 | 3.6±5.1 | 0.68 | −12.2±54.4 | −3.1, +9.2 | 0 |
| 25-hydroxyvitamin D3 (ng/ml) | 25.4±10.4 | 26.0±10.9 | 0.62 | −2.8±18.3 | −1.6, +5.3 | 0 |
| Total 25-hydroxyvitamin D (ng/ml) | 28.9±11.3 | 29.5±11.4 | 0.58 | −3.1±18.3 | −3.2, +5.0 | 0 |
Paired t test comparing arterial and venous concentrations.
The most striking difference was observed for PTH, which underwent a mean single-pass renal elimination of 44.2% with a relatively tight distribution (IQR, 38.0%–50.7%). No PTH was detected in the urine and the urinary concentrations of vitamin D metabolites were below the assay limits of detection. The mean single-pass renal elimination of FGF23 was lower than that of PTH with a wider variation across study participants (mean 17.1%; IQR, 0.9; 30.0%). The mean FGF23 concentration in urine was 15.9±15.2 pg/ml.
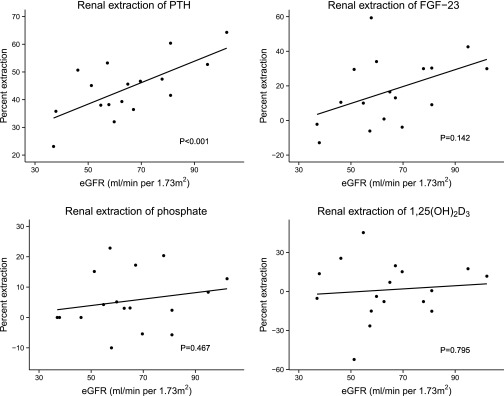
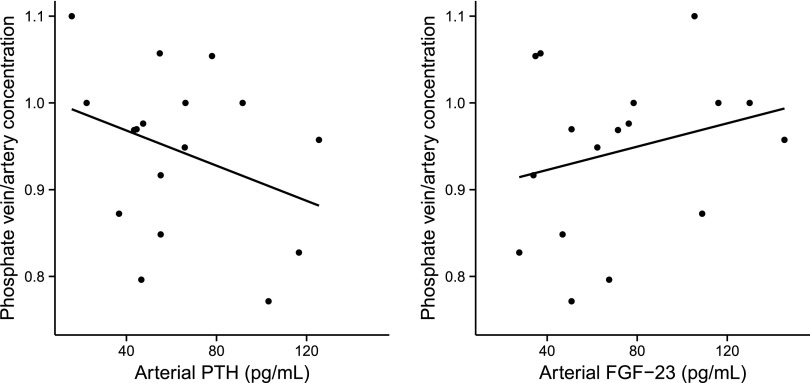
Lower eGFR was associated with a lower proportionate renal extraction of PTH (Figure 1). There was a tendency toward similar associations of lower eGFR with lower extractions of FGF23 and phosphate; however, these associations were not statistically significant. The Pearson correlation coefficients of eGFR with PTH, FGF23, phosphate, and 1,25(OH)2D were: 0.68 (P=0.01), 0.46 (P<0.01), 0.21 (P=0.43), and 0.10 (P<0.71), respectively. There was a trend of higher PTH levels with progressively lower renal vein phosphate concentrations relative to those in the aorta (Figure 2A; P=0.19). In contrast, there was a trend of higher FGF23 concentrations with a higher renal vein to artery ratio of phosphate. (Figure 2B; P=0.32). Neither association was statistically significant.
Figure 1.
Renal extraction of mineral metabolism markers versus estimated glomerular filtration rate. The x-axis depicts the eGFR. The y axis depicts the estimated single-pass renal elimination of each mineral metabolism marker. Raw data and best-fit unadjusted linear regression lines are presented along with P values for linear regression.
Figure 2.
Arterial PTH and FGF23 by phosphate vein/artery concentrations.
Discussion
In this study we found a relatively large (44%) single-pass renal extraction of PTH using blood samples directly obtained from the abdominal aorta and renal vein. In contrast, differences in FGF23 concentrations across the aorta and renal vein were highly variable among study participants, with an average renal extraction of 17%. Mean concentrations of vitamin D metabolites were not statistically different in the aorta versus renal vein. The average renal extraction of creatinine was 22.1%, which is consistent with a filtration marker, validates the experiment, and demonstrates minimal creatinine secretion among this study sample. These findings imply substantial nonglomerular clearance of PTH and suggest that kidney impairment itself could contribute to PTH excess in CKD.
The renal clearance of mineral metabolism markers is largely unknown. Some small human studies have investigated PTH clearance.7,8 Among nine hyperparathyroid patients, the mean extraction of PTH was 44% across the liver; 34% across the kidney, and 16% across the leg.7 Among ten hyperparathyroid patients, the renal extraction of PTH was 47%.8 Our renal extraction of PTH (44%) is in agreement with these previous studies.
Experimental dog models have studied several mechanisms of PTH extraction. After ureteral obstruction, which halts glomerular filtration, mean creatinine extraction by the dog kidney decreased from 20% to 4%, whereas mean PTH extraction only decreased from 22% to 15%, indicating that the kidney continues to extract PTH from the circulation when the glomerular filtration rate is decreased by ureteral obstruction.6 Other experiments suggest that PTH is degraded to a number of smaller fragments by the kidney. In isolated perfused dog kidneys, circulating PTH is hydrolyzed to an inactive form.4,6 Most likely, this partial hydrolysis takes place at the peritubular membranes where PTH receptors are located. Binding to these receptors is associated with degradation of the intact hormone to amino- and carboxyl-terminal fragments,9 which could explain why the renal extraction of PTH is greater than filtration alone. These experimental studies give valuable insight into renal extraction and PTH catabolism pathways, although it should be noted that these experiments should be interpreted with caution because a potential species difference might occur. Taken together, these results indicate that the kidney is an important organ in metabolizing PTH.5
To our knowledge, no human study has investigated the renal clearance of FGF23 by single-pass renal extraction. In a small study among ESRD patients (n=10), FGF23 clearance was measured based on plasma FGF23, urinary or dialysate FGF23, and 24 h urine volume. The median renal FGF23 clearance was 0.2 (IQR, 0.1–0.3) ml/min.10 In four dialysis patients, urinary FGF23 levels ranged from 750 to 10,790 RU/ml, suggesting renal clearance of FGF23 with intact FGF23 molecule present in the urine.11 There were four participants in this study who had slightly greater FGF23 concentrations in the renal vein compared with the aorta (2%–6% greater). These differences are within assay variability for singlicate runs of intact FGF23 in our laboratory (coefficient of variation 6.7%–12.4%).
Lower kidney function could contribute to metabolite “accumulation” through reduced cellular uptake, or impaired degradation, as has been described for other proteins.12,13 The proportion of C-terminal FGF23 fragments relative to intact FGF23 increases with the severity of CKD.14 However, the relative excess of iFGF23 compared with cFGF23 fragments in advanced CKD could represent increased production of iFGF23 production by bone if the catabolism of iFGF23 were to occur at a fixed rate. The positive relationship between eGFR and renal FGF23 excretion as found by our study indicates that FGF23 is at least partly cleared by the kidney and could explain the rapid decrease in FGF23 concentrations in some renal transplant recipients.15,16 The clearance of FGF23 by cellular uptake and/or urinary excretion should be studied to understand the actions of the kidney on FGF23 concentrations to develop therapeutic approaches to treat diseases caused by excess FGF23.
It is likely that single-pass renal extraction and production of vitamin D metabolites is too small to be accurately quantified in this study. Metabolic production and clearance rates of vitamin D metabolites are slow, resulting in circulating half-lives of hours to weeks,17 in contrast to the rapid clearance and short half-lives of PTH and FGF23.18,19
There were three study participants with higher phosphate concentrations in the renal vein compared with the aorta (vein to artery ratio 1.05%–1.10%). These differences are slightly above the assay variability for singlicate runs of phosphate (approximately 5%). It is possible that minute-to-minute variation in circulating phosphate concentrations in conjunction with small differences in venous and arterial blood sampling times could explain these findings. Intrinsic renal production of phosphate is unlikely, but cannot be ruled out.
This study has several limitations. Our study population of individuals undergoing invasive heart catheterization was rather small (n=17) and may not be generalizable to other populations. Our study population was characterized by normal or only modestly impaired kidney function with relatively normal or only modestly abnormal concentrations of mineral metabolism markers. The impact of renal extraction could be different in more advanced CKD populations with more severe disturbances of mineral metabolism markers and lower eGFR. Moreover, all study participants were undergoing an elective cardiac catheterization procedure, diminishing the applicability of study findings. These results should therefore be replicated to validate our findings and extend generalizability to other groups. We measured PTH using a second-generation immunoassay that is specific to whole-molecule (1,84) PTH. Although this assay cross-reacts with exogenous 7,84 PTH fragments synthesized in the laboratory, there is no evidence that 7,84 PTH fragments circulate in normal individuals. It is possible that PTH is cleaved into smaller fragments that are present in the urine or renal vein and were therefore not detected in this study. Similarly, our findings for FGF23 pertain only to the intact molecule because we did not measure C-terminal FGF23 fragments in this study. Furthermore, urine FGF23 measurements were performed in the absence of gold-standard validation studies and may be subject to discrepancies due to inferring metabolites in urine. Finally, we did not measure other bone-related markers that are also known to be important in mineral metabolism disorders of CKD.
Our study of individuals undergoing invasive heart catheterization demonstrates that the mean concentrations of PTH, FGF23, and phosphate were significantly lower in the renal vein compared with the renal artery. Lower eGFR was associated with reduced single-pass renal extraction of PTH. The renal extraction of PTH exceeded that of creatinine, implying a non-GFR–dependent mechanism. This study provides further evidence of the importance of the kidney in PTH metabolism and a possible role for the kidney in FGF23 clearance.
Concise Methods
Study Population
We obtained blood and urine samples from 20 participants undergoing simultaneous right and left heart catheterization at the Massachusetts General Hospital Cardiac Catheterization Laboratory.20 The main indications for the procedure were aortic valve disease (n=8), mitral valve disease (n=3), unexplained dyspnea (n=4), followed by miscellaneous causes. In addition to routine clinical care, the protocol included insertion of a Judkins right catheter into the ostium of a renal vein, with plasma sampling from this catheter and from a catheter positioned in the abdominal aorta at the level of the renal arteries prior to catheterization and before administration of iodinated contrast medium. The renal vein sample was collected first, followed immediately by the arterial sample.
We excluded two participants for whom there was technical difficulty in catheter placement and one participant who had ESRD, leaving 17 participants for analysis. All participants were fasting at the time of their procedure. First morning voided urine was obtained from the final seven study participants. The study protocol was approved by the Massachusetts General Hospital Institutional Review Board and was in adherence to the Declaration of Helsinki. All participants provided written informed consent.
Mineral Metabolism Markers
Serum and urine samples were shipped on dry ice to The University of Washington Nutrition Obesity Research Center, which measured all of the metabolites in this study. Serum PTH was measured using a two-site immunoassay (Beckman Coulter, Brea, CA). This assay captures 100% of the full-length PTH molecule (1–84) and 77% of 7–84 PTH fragments; other PTH fragments are not detected by this assay.21 The interassay coefficient of variation (COV) for PTH is 6.7% and the minimal detectable concentration is 1.0 pg/ml. We measured FGF23 concentrations using the Kainos immunoassay, which detects the full-length, biologically intact FGF23 molecule via mid-molecule and distal epitopes. The COV for FGF23 ranges between 6.7% and 12.4% based on singlicate high and low control samples. We measured serum 1,25-dihydroxyvitmain D3 [1,25(OH)2D3], 1,25-dihydroxyvitmain D2 [1,25(OH)2D2], 24,25-dihydroxyvitamin D3 [24,25(OH)2D3], 25-hydroxyvitamin D2 [25(OH)D2], and 25-hydroxyvitamin D3 [25(OH)D3] using liquid chromatography-mass spectroscopy on a Quattro Micro mass spectrometer (Water, Milford, T.3 Interassay COVs across a range of measured concentrations of 1,25(OH)2D3 are 3.7%–10.2% and the minimum detectable concentration is 3.4 pg/ml. For 24,25(OH)2D3 the interassay COV is 2.6% and the minimum detectable concentration is 0.06 ng/ml. The interassay COVs for 25(OH)D2 and 25(OH)D3 are 4.4%, with lower limits of detection of 0.5 and 2.0 ng/ml, respectively. Serum and urine phosphate and creatinine were measured using a timed-rate colorimetry reaction on a Beckman Coulter UniCel DxC instrument. Urine FGF23 concentrations were measured according to the manufacturer’s instructions using intact FGF23 (Kainos assay).
We defined total 25(OH)D as 25(OH)D2+25(OH)D3 and we defined total 1,25(OH)2D as 1,25(OH)2D2+25(OH)2D3. We calculate eGFR from the 2009 CKD-EPI equation22 using renal artery creatinine concentrations, because these values were closest to those abstracted from the medical chart. In contrast, renal vein creatinine values were systematically lower than the medical chart values.
Statistical Analyses
We used two-tailed paired t tests to compare renal arterial versus renal vein concentrations of each metabolite. We calculated the single-pass renal elimination of each metabolite as follows:
 |
Positive renal elimination values indicate the proportionate removal of a metabolite via a single pass through the kidneys. In contrast, negative renal elimination values indicate net addition of a metabolite through the kidneys. Because GFR normally represents approximately 20% of the renal plasma flow, the expected renal extraction of a substance that is freely filtered at the glomerulus and not reabsorbed, secreted, metabolized, or synthesized within the kidneys should be about 20%. We used scatter plots, Pearson correlation, and linear regression to estimate associations of eGFR with the renal elimination of metabolites. We conducted analyses with R software version 3.1.1 (R Foundation Statistical Computing) and STATA 13.1 (College Station, TX).
Disclosure
Dr. de Boer reports receiving research grant funding from Abbvie. Dr. Kestenbaum reports receiving consulting fees from Keryx Biopharmaceuticals Inc.
Acknowledgments
This research was supported by the Niels Stensen Fellowship 2014, the Extramural Grant Program by Satellite Healthcare, and NIH grants K08-DK090142 and R01-DK099199.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Ureña-Torres P, Metzger M, Haymann JP, Karras A, Boffa JJ, Flamant M, Vrtovsnik F, Gauci C, Froissart M, Houillier P, Stengel B, NephroTest Study Group : Association of kidney function, vitamin D deficiency, and circulating markers of mineral and bone disorders in CKD. Am J Kidney Dis 58: 544–553, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Silver J, Naveh-Many T: FGF-23 and secondary hyperparathyroidism in chronic kidney disease. Nat Rev Nephrol 9: 641–649, 2013 [DOI] [PubMed] [Google Scholar]
- 3.de Boer IH, Sachs MC, Chonchol M, Himmelfarb J, Hoofnagle AN, Ix JH, Kremsdorf RA, Lin YS, Mehrotra R, Robinson-Cohen C, Siscovick DS, Steffes MW, Thummel KE, Tracy RP, Wang Z, Kestenbaum B: Estimated GFR and circulating 24,25-dihydroxyvitamin D3 concentration: a participant-level analysis of 5 cohort studies and clinical trials. Am J Kidney Dis 64: 187–197, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hruska KA, Martin K, Mennes P, Greenwalt A, Anderson C, Klahr S, Slatopolsky E: Degradation of parathyroid hormone and fragment production by the isolated perfused dog kidney. The effect of glomerular filtration rate and perfusate CA++ concentrations. J Clin Invest 60: 501–510, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kau ST, Maack T: Transport and catabolism of parathyroid hormone in isolated rat kidney. Am J Physiol 233: F445–F454, 1977 [DOI] [PubMed] [Google Scholar]
- 6.Martin KJ, Hruska KA, Lewis J, Anderson C, Slatopolsky E: The renal handling of parathyroid hormone. Role of peritubular uptake and glomerular filtration. J Clin Invest 60: 808–814, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oldham SB, Finck EJ, Singer FR: Parathyroid hormone clearance in man. Metabolism 27: 993–1001, 1978 [DOI] [PubMed] [Google Scholar]
- 8.Corvilain J, Manderlier T, Struyven J, Fuss M, Bergans A, Nijs N, Brauman H: Metabolism of human PTH by the kidney and the liver. Horm Metab Res 9: 239–242, 1977 [DOI] [PubMed] [Google Scholar]
- 9.Silva BC, Costa AG, Cusano NE, Kousteni S, Bilezikian JP: Catabolic and anabolic actions of parathyroid hormone on the skeleton. J Endocrinol Invest 34: 801–810, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isakova T, Xie H, Barchi-Chung A, Vargas G, Sowden N, Houston J, Wahl P, Lundquist A, Epstein M, Smith K, Contreras G, Ortega L, Lenz O, Briones P, Egbert P, Ikizler TA, Jueppner H, Wolf M: Fibroblast growth factor 23 in patients undergoing peritoneal dialysis. Clin J Am Soc Nephrol 6: 2688–2695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsson T, Nisbeth U, Ljunggren O, Jüppner H, Jonsson KB: Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 64: 2272–2279, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Waldmann TA, Strober W, Mogielnicki RP: The renal handling of low molecular weight proteins. II. Disorders of serum protein catabolism in patients with tubular proteinuria, the nephrotic syndrome, or uremia. J Clin Invest 51: 2162–2174, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maack T, Johnson V, Kau ST, Figueiredo J, Sigulem D: Renal filtration, transport, and metabolism of low-molecular-weight proteins: a review. Kidney Int 16: 251–270, 1979 [DOI] [PubMed] [Google Scholar]
- 14.Smith ER, Cai MM, McMahon LP, Holt SG: Biological variability of plasma intact and C-terminal FGF23 measurements. J Clin Endocrinol Metab 97: 3357–3365, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Pande S, Ritter CS, Rothstein M, Wiesen K, Vassiliadis J, Kumar R, Schiavi SC, Slatapolsky E, Brown AJ: FGF-23 and sFRP-4 in chronic kidney disease and post-renal transplantation. Nephron, Physiol 104: 23–32, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Economidou D, Dovas S, Papagianni A, Pateinakis P, Memmos D: FGF-23 Levels before and after Renal Transplantation. J Transplant 2009;2009:379082 [DOI] [PMC free article] [PubMed]
- 17.Vieth R: Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr 69: 842–856, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Yamashita H, Gao P, Cantor T, Futata T, Murakami T, Uchino S, Watanabe S, Kawamoto H, Fukagawa M, Noguchi S: Large carboxy-terminal parathyroid hormone (PTH) fragment with a relatively longer half-life than 1-84 PTH is secreted directly from the parathyroid gland in humans. Eur J Endocrinol 149: 301–306, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Khosravi A, Cutler CM, Kelly MH, Chang R, Royal RE, Sherry RM, Wodajo FM, Fedarko NS, Collins MT: Determination of the elimination half-life of fibroblast growth factor-23. J Clin Endocrinol Metab 92: 2374–2377, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Rhee EP, Clish CB, Ghorbani A, Larson MG, Elmariah S, McCabe E, Yang Q, Cheng S, Pierce K, Deik A, Souza AL, Farrell L, Domos C, Yeh RW, Palacios I, Rosenfield K, Vasan RS, Florez JC, Wang TJ, Fox CS, Gerszten RE: A combined epidemiologic and metabolomic approach improves CKD prediction. J Am Soc Nephrol 24: 1330–1338, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Access Immunoassay Systems, Access Intact PTH, Beckman Coulter, Inc. Ref: A16972. 2011
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]