Abstract
Immune complex tubulointerstitial nephritis due to antibodies to brush border antigens of the proximal tubule has been demonstrated experimentally and rarely in humans. Our patient developed ESRD and early recurrence after transplantation. IgG and C3 deposits were conspicuous in the tubular basement membrane of proximal tubules, corresponding to deposits observed by electron microscopy. Rare subepithelial deposits were found in the glomeruli. The patient had no evidence of SLE and had normal complement levels. Serum samples from the patient reacted with the brush border of normal human kidney, in contrast with the negative results with 20 control serum samples. Preliminary characterization of the brush border target antigen excluded megalin, CD10, and maltase. We postulate that antibodies to brush border antigens cause direct epithelial injury, accumulate in the tubular basement membrane, and elicit an interstitial inflammatory response.
Keywords: immune complex, tubulointerstitial, brush border
Clinical History
A 73-year-old man presented with nausea, vomiting, and progressive renal failure. His serum creatinine had increased from 1.3 mg/dl to 15.2 mg/dl in the 10 months before admission. He had a history of coronary artery disease, diabetes, and hypertension, and he was taking multiple medications including omeprazole, lisinopril, metformin, simvastatin, metoprolol, aspirin, and insulin. On admission, his BP was 148/78 mmHg. His other vital signs were normal and his physical examination was unremarkable. He had no prior history of autoimmune disease and no family history of autoimmune or renal diseases.
Urinalysis showed protein (2+), blood (1+), 3–5 white blood cells per high-power field, and no casts. His hemoglobin was 12.6 g/L, although white blood cell counts and platelets were normal. Serologies were negative, including ANCA, anti–glomerular basement membrane (GBM), anti-nuclear antibody, anti–double-stranded DNA, and hepatitis B and C. C3 and C4 levels were normal. An ultrasound showed no evidence of a mass, obstruction, or stenosis.
Kidney Biopsy
Ten histologically normal glomeruli were present with no endocapillary hypercellularity or GBM abnormality. There was prominent diffuse tubular injury manifested by loss of brush borders, blebs, vacuoles, and regenerative changes. An interstitial infiltrate of lymphocytes, plasma cells, and a few eosinophils affected 50%–60% of the cortex (Figure 1), associated with focal tubulitis. Interstitial fibrosis and tubular atrophy were diffuse and present in 70% of the cortex. No giant cells were seen. An artery showed mild intimal fibrosis. Few IgG4-positive plasma cells were detected in the interstitium (mean of 2 per high-power field).
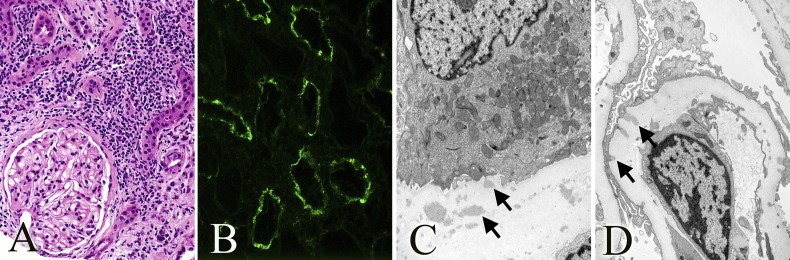
Figure 1.
Native kidney. (A) Mononuclear inflammatory interstitial infiltrate with few admixed neutrophils and eosinophils, tubular atrophy, and normal-looking glomerulus (hematoxylin and eosin staining). (B) Granular IgG staining of TBMs. (C) Amorphous electron-dense deposits along the TBM. (D) Penetrating subepithelial electron-dense deposits along the GBM. Original magnification, ×400 in A and B; ×8900 in C and D.
Immunofluorescence (IF) showed widespread granular deposits along the proximal tubular basement membrane (TBM), which stained for IgG (3+), C3 (3+), and equally for κ and λ (Figure 1). The TBM deposits stained with IgG1 (widespread 3+), IgG2 (focal 2+), and IgG4 (widespread 3+). IgG3 was negative. The glomerular deposits were sparse, and they showed very segmental staining for IgG and did not stain for the phospholipase-A2 receptor.
Ultrastructural analysis showed widespread amorphous electron-dense deposits within the TBM, sometimes abutting the basal plasma membrane of the tubular cell (Figure 1). Scattered penetrating subepithelial deposits were present in a small minority of glomerular capillaries without spike formation (Figure 1). The GBM was diffusely but mildly thickened (harmonic mean thickness of 602 nm), indicating early diabetic glomerulopathy.
These findings were interpreted as immune complex tubulointerstitial nephritis (TIN) with minor involvement of the glomerulus resembling early membranous GN.
Clinical Follow-Up
The patient was treated with steroids but remained hemodialysis dependent. He received a deceased donor allograft kidney 4 years later. The immediate post-transplant course was unremarkable and he was maintained on tacrolimus, mycophenolate mofetil, and prednisone.
Seven weeks later, the patient again developed nausea and vomiting and his serum creatinine level increased (2 mg/dl). Lupus serologies remained negative and complement levels were normal. An allograft biopsy showed a sparse inflammatory infiltrate of about 5% of the cortex, composed of lymphocytes, occasional neutrophils, and rare eosinophils. Loss of brush borders, dilation, and protein casts were focally present, but there was no tubulitis. Glomeruli and vessels were unremarkable. IF was similar to the native biopsy, with diffuse granular TBM deposits that stained for IgG (4+), C3 (4+), and C4d (4+). The glomeruli were negative. C4d was negative in peritubular capillaries. The polyoma stain was negative (SV40 large T antigen). The TBM deposits stained for IgG1 (widespread 3+) and were negative for IgG2, IgG3, and IgG4. The significance of the difference in IgG subclasses in the native and allograft biopsies is not clear. Amorphous electron-dense deposits were detected in the TBM abutting the proximal tubule basal membrane, similar to the native kidney biopsy. These findings were interpreted as recurrent immune complex TIN.
The patient’s pretransplant and post-transplant sera were tested for anti–brush border autoantibodies (ABBAs) by indirect IF on cryostat sections of normal kidneys at a 1:5 dilution. Strong and selective binding of IgG to the brush border of proximal tubules was evident without reactivity to the TBM (Figure 2). Control normal human sera (n=20) did not stain brush borders under the same conditions. To seek the antigenic target, double staining with the patient’s serum was repeated combined with antibodies to megalin (Figure 3), CD10, or maltase. No colocalization was present (data not shown).
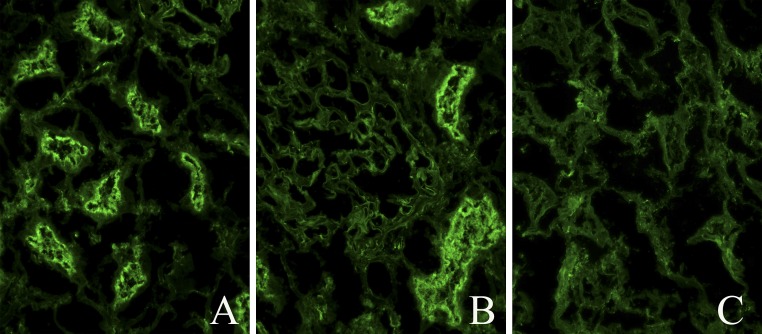
Figure 2.
Indirect IF of the patient’s serum on normal human kidney. (A) IgG staining of proximal tubule brush borders. (B) Proximal tubule brush border IgG staining and negative glomerular staining. (C) Control normal serum on normal human kidney.
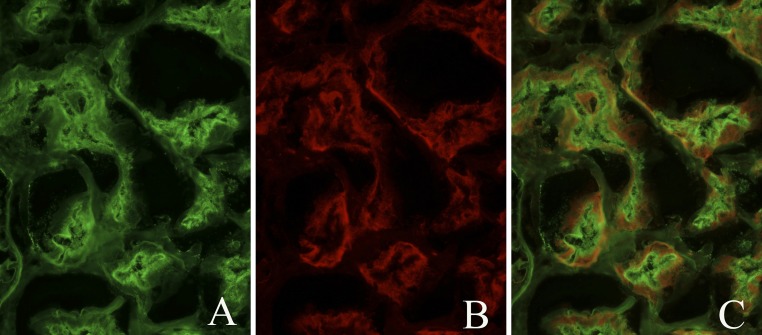
Figure 3.
Patient IgG-megalin on normal human kidney, colocalization by IF. (A) Patient IgG staining on proximal tubule brush borders. (B) Megalin staining on proximal tubule brush borders. (C) No evident IgG-megalin colocalization.
Plasma exchange was initiated, along with intravenous Ig and rituximab, without dramatic benefit. At 45 weeks post-transplant, the patient’s serum creatinine was 3 mg/dl. A repeat allograft biopsy showed progression of the TIN, now involving 30% of the cortex, loss of tubular brush borders, and flattened epithelial cells and persistent TBM deposits by IF and electron microscopy (Figure 4). C4d was negative in peritubular capillaries and SV40 was negative. Rare subepithelial glomerular deposits were identified. The patient received methylprednisolone pulses, rituximab, and later belatacept. Serum creatinine levels slowly increased to 3.1 mg/dl 2 years post-transplant.
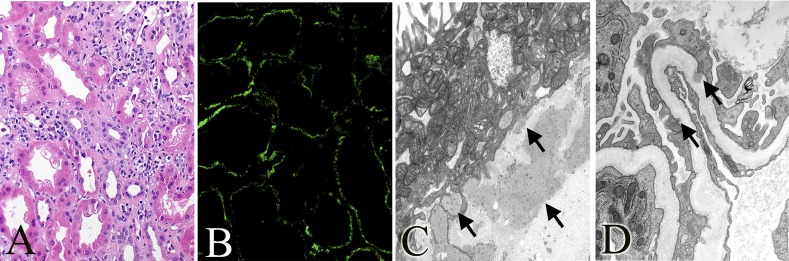
Figure 4.
Allograft kidney. (A) Tubular epithelial blebs, vacuolization, focal tubulitis, and mononuclear inflammatory interstitial infiltrate, 45 weeks post-transplant (hematoxylin and eosin staining). (B) Granular IgG staining of TBMs, 45 weeks post-transplant. (C) Large amorphous electron-dense deposits along the TBM, 7 weeks post-transplant; similar deposits are present at 45 weeks. (D) Rare amorphous subepithelial electron-dense deposits along the GBM, 45 weeks post-transplant. Original magnification, ×400 in A and B; ×14,000 in C; ×18,000 in D.
Antibody titers were determined by indirect IF using sera from pretransplant and post-transplant immunosuppressive treatment time points. The titer before kidney transplantation was >1:960. Before plasma exchange, the titer was noted at =1:240 and was at =1:120 ten weeks after plasma exchange and immunosuppressive therapy (intravenous Ig, rituximab, tacrolimus, and steroids). There was an apparent decrease in antibody titer after transplantation, along with some decrease in titer after plasma exchange and immunosuppressive therapy.
Discussion
There are several causes of immune complex–mediated TIN in humans that should be considered in the differential diagnosis (Table 1).
Table 1.
Pathologic characteristics and outcome of immune complex tubulointerstitial nephritides
| Disease | Light Microscopy | IF | Electron Microscopy | Glomerular Involvement | ABBA | Outcome |
|---|---|---|---|---|---|---|
| Lupus nephritis | Tubulitis and interstitial inflammation | Granular TBM for IgGs and complement | Electron-dense TBM deposits | Rarely with minimal glomerular involvement | − | Partial to complete response to high-dose corticosteroids1,2 |
| IgG4-related systemic disease | Storiform fibrosis with IgG4+plasma cell–rich infiltrate | Granular TBM for IgG and C3, commonly IgG4 subset | Electron-dense TBM deposits | With or without membranous glomerulopathy | − | General response to steroids; refractory cases may respond to rituximab3 |
| Idiopathic hypocomplementemic TIN | Tubulitis and interstitial inflammation | Granular TBM for IgG and C3 | Electron-dense TBM deposits | − | − | Partial to complete response to immunosuppression4 |
| Drug-induced TIN with TBM immune complex deposits | Tubulitis and interstitial inflammation with eosinophils with or without neutrophils | Granular TBM for IgG and C3 | Electron-dense TBM deposits | − | − | Responsive to steroids5 |
| Polyomavirus nephropathy | Viral cytopathic changes | Granular TBM staining for C4d, IgG, and C3 | Electron-dense TBM deposits | − | − | Responsive to decrease in immunosuppression plus cidofovir, leflunomide, or intravenous Ig8 |
| ABBA-TIN | Tubular injury and interstitial inflammation | Granular TBM staining for C4d, IgG, and C3 | Electron-dense TBM deposits | Minimal subepithelial deposits | + | Developed ESRD over years; recurred in transplant |
Lupus nephritis is the most common cause of immune complex deposition in the TBM, occurring in about 60% of lupus GN. Isolated TIN in SLE has also been reported.1,2
IgG4-related systemic disease (IgG4-RSD) typically presents with multiorgan involvement, including autoimmune pancreatitis, inflammatory bowel disease, sclerosing cholangitis, and/or sialadenitis, as well as TIN. Hypergammaglobulinemia with elevated IgG4 levels is usually present and 50% of patients have hypocomplementemia. Renal biopsy shows IgG4+ plasma cell–rich infiltrate, storiform fibrosis, and granular TBM immune complex deposits for IgG and C3. IgG4-RSD is steroid responsive and rarely causes renal failure.3 The antigenic target remains uncertain, but ABBAs have not been reported.
Idiopathic hypocomplementemic TIN is manifested by granular TBM deposits of IgG and C3 in association with hypocomplementemia. This preferential TBM deposition has been reported in eight cases, all in the setting of low complement levels and not associated with SLE or Sjögren’s syndrome. ABBAs have not been reported in this setting.4
Drug-induced TIN with TBM immune complex deposition has been rarely documented in association with nonsteroidal anti-inflammatory drugs.5 Polyclonal IgG and C3 TBM deposits are present without glomerular deposits. Two cases showed giant cell tubulitis with TBM deposits.6 In both settings, the deposits were postulated to arise from antidrug antibodies or elicited autoantibodies.
Polyomavirus nephropathy can cause focal granular TBM deposits of IgG, C3, and C4d in addition to TIN and viral cytopathic changes, most often seen in allograft kidneys. The nature of the antigen is uncertain. These have been reported to react with one antiserum but not with antibodies to major capsid protein, VP1, minor capsid proteins, VP2 and VP3, or large T antigen.7,8
The clinical, laboratory, and biopsy findings in our patient rule out these more common causes of immune complex TIN. In this patient, there was no evidence of lupus, hypocomplementemia, or IgG4-RSD, and there was no established association with drugs or virus and no response to steroids. We therefore believe that this is a distinctive form of primary immune complex TIN associated with ABBAs.
We were able to find only one somewhat similar case in the literature.9 A patient with myasthenia gravis developed ARF owing to TIN, granular IgG and C3 deposits in the proximal TBM, and epimembranous deposits in the GBM. ABBA was documented in the patient’s serum and in the eluate from the post mortem kidney. ABBAs have also been detected rarely in membranous GN, but TBM deposits were not noted.10
In the older literature, ABBAs have been reported in Crohn’s disease and in about 10% of sera submitted for autoantibody screening; however, these were not kidney specific and were not associated with renal disease.11 To address the possibility that the ABBA was an incidental finding, we tested 20 control sera from samples submitted for either ANCA or anti-GBM assays. These were uniformly negative for ABBA, although 15% had anti-nuclear antibodies. Hence, we believe the ABBAs detected in this patient are a distinctive finding.
An experimental model for ABBA-TIN was reported by Klassen et al. in 1977. They immunized rabbits with nonglomerular components of the rabbit kidney, which led to tubular injury, interstitial fibrosis, and focal lymphocytic infiltrates with granular IgG and C3 deposition along the TBM of proximal tubules.12 Antibodies eluted from diseased kidneys reacted with the proximal tubule brush border and the TBM deposits.12 The nature of the antigen was not further characterized. This study may provide insight into the pathophysiology of ABBA in our current case. However, the trigger of the ABBA in our case is unknown.
Another experimental model is Heymann nephritis. Sera from these rats react with megalin in the brush border of proximal tubules and podocytes.13 Passive transfer of antibodies causes deposition of antibodies in vivo along the GBM in an epimembranous pattern, the proximal tubule brush border and deposit in the TBM,14 with associated disruption of the normal architecture of the proximal tubules.14,15 In humans, megalin is expressed in the brush border but not in podocytes. ABBAs have also been noted in murine chronic graft-versus-host disease.16
The native kidneys in this patient failed because of progressive tubulointerstitial inflammation and scarring, which suggests persistent antibody formation. This hypothesis was supported by persistence of the ABBA and early recurrence in the allograft. We postulate that IgG autoantibody to an unknown antigen on the proximal tubule brush border likely caused direct epithelial cell injury, accumulated as in situ immune complexes and caused interstitial inflammation and fibrosis. Rare subepithelial deposits in the native and transplant kidney suggest that the antigen is expressed to a minor degree by the podocyte.
Disclosures
None.
Acknowledgments
This work was made possible through an International Society of Nephrology Fellowship Grant to I.A.R.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Dhingra S, Qureshi R, Abdellatif A, Gaber LW, Truong LD: Tubulointerstitial nephritis in systemic lupus erythematosus: Innocent bystander or ominous presage. Histol Histopathol 29: 553–565, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Singh AK, Ucci A, Madias NE: Predominant tubulointerstitial lupus nephritis. Am J Kidney Dis 27: 273–278, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Cornell LD: IgG4-related kidney disease. Curr Opin Nephrol Hypertens 21: 279–288, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Kambham N, Markowitz GS, Tanji N, Mansukhani MM, Orazi A, D’Agati VD: Idiopathic hypocomplementemic interstitial nephritis with extensive tubulointerstitial deposits. Am J Kidney Dis 37: 388–399, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Dixit MP, Nguyen C, Carson T, Guedes B, Dixit NM, Bell JM, Wang Y: Non-steroidal anti-inflammatory drugs-associated acute interstitial nephritis with granular tubular basement membrane deposits. Pediatr Nephrol 23: 145–148, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Chang A, Peutz-Kootstra CJ, Kowalewska J, Logar CM, Gitomer JJ, Davis CL, Shankland SJ, Alpers CE, Smith KD: Giant cell tubulitis with tubular basement membrane immune deposits: A report of two cases after cardiac valve replacement surgery. Clin J Am Soc Nephrol 1: 920–924, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Hever A, Nast CC: Polyoma virus nephropathy with simian virus 40 antigen-containing tubular basement membrane immune complex deposition. Hum Pathol 39: 73–79, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Bracamonte E, Leca N, Smith KD, Nicosia RF, Nickeleit V, Kendrick E, Furmanczyk PS, Davis CL, Alpers CE, Kowalewska J: Tubular basement membrane immune deposits in association with BK polyomavirus nephropathy. Am J Transplant 7: 1552–1560, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Morrison EB, Kozlowski EJ, McPhaul JJ, Jr: Primary tubulointerstitial nephritis caused by antibodies to proximal tubular antigens. Am J Clin Pathol 75: 602–609, 1981 [DOI] [PubMed] [Google Scholar]
- 10.González-Cabrero J, de Nicolas R, Ortíz A, Mampaso F, Hernando L, Egido J: Presence of circulating antibodies against brush border antigens (Fx1A) in a patient with membranous nephropathy and bilateral pyeloureteral stenosis. Comparison with idiopathic membranous nephropathy. Nephrol Dial Transplant 7: 293–299, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Skogh T, Heuman R, Tagesson C: Anti-brush border antibodies (ABBA) in Crohn’s disease. J Clin Lab Immunol 9: 147–150, 1982 [PubMed] [Google Scholar]
- 12.Klassen J, Milgrom FM, McCluskey RT: Studies of the antigens involved in an immunologic renal tubular lesion in rabbits. Am J Pathol 88: 135–144, 1977 [PMC free article] [PubMed] [Google Scholar]
- 13.Kerjaschki D, Farquhar MG: Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J Exp Med 157: 667–686, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendrick DL, Noble B, Brentjens JR, Andres GA: Antibody-mediated injury to proximal tubules in Heymann nephritis. Kidney Int 18: 328–343, 1980 [DOI] [PubMed] [Google Scholar]
- 15.Noble B, Andres GA, Brentjens JR: Passively transferred anti-brush border antibodies induce injury of proximal tubules in the absence of complement. Clin Exp Immunol 56: 281–288, 1984 [PMC free article] [PubMed] [Google Scholar]
- 16.Bruijn JA, Hogendoorn PC, Corver WE, van den Broek LJ, Hoedemaeker PJ, Fleuren GJ: Pathogenesis of experimental lupus nephritis: A role for anti-basement membrane and anti-tubular brush border antibodies in murine chronic graft-versus-host disease. Clin Exp Immunol 79: 115–122, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]