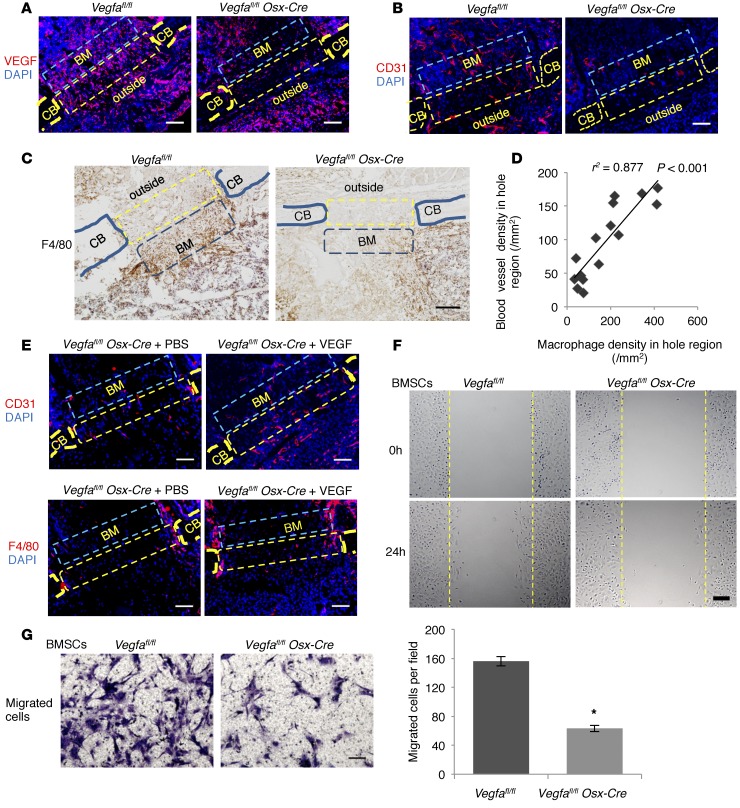
Figure 6. Osteoblast-derived VEGF stimulates macrophage-related angiogenesis and migration of BM cells.
(A) Increased anti-VEGF staining in hole (yellow rectangles) (17.6% ± 4.8%) and BM (blue rectangles) (20.3% ± 4.9%) of Vegfafl/fl compared with Vegfa CKO mice (4.2% ± 1.2% and 6.6% ± 2.0%) at PSD3; n= 5–6, P < 0.05. No significant differences outside hole (10.2% ± 2.9% vs. 9.4% ± 2.0%) (B) High anti-CD31 staining in hole (163/mm2 ± 5/mm2), adjacent BM (263/mm2 ± 18/mm2), and outside area (164/mm2 ± 26/mm2) of Vegfafl/fl compared with Vegfa CKO mice (58/mm2 ± 12/mm2, 65/mm2 ± 21/mm2, and 66/mm2 ± 17/mm2) at PSD3; n = 4–7, P < 0.05. (C) High density of F4/80+ macrophages in hole (321/mm2 ± 46/mm2) and BM (366/mm2 ± 69/mm2) of Vegfafl/fl compared with Vegfa CKO mice (82/mm2 ± 19/mm2 and 143/mm2 ± 26/mm2) at PSD3; n = 5–6, P < 0.01 (hole); P < 0.05 (BM); no difference outside (217/mm2 ± 38/mm2 vs. 166/mm2 ± 41/mm2). (D) High correlation between blood vessel and macrophage densities in hole. (E) Treating Vegfa CKO mice with 0.1 μg VEGF increases blood vessel density in hole (151/mm2 ± 7/mm2) and BM (115/mm2 ± 9/mm2) and macrophage density in hole (205/mm2 ± 34/mm2) at PSD5, compared with PBS (91/mm2 ± 12/mm2, 50/mm2 ± 6/mm2, and 87/mm2 ± 9/mm2); P < 0.05. Macrophage density in BM not affected (140/mm2 ± 25/mm2 vs. 94/mm2 ± 13/mm2). (F) In vitro wound closure; stippled lines indicate wound edges at time 0. Higher wound closure rate (μm/h) in BMSCs of Vegfafl/fl (8.1 ± 0.7 μm/h) than Vegfa CKO mice (5.1 ± 0.3 μm/h); n = 3, P < 0.05. (G) Transwell migration assay. After 14 hours, more migrated BMSCs from Vegfafl/fl than Vegfa CKO mice; n= 3, *P < 0.01. Scale bars: 50 μm (G), 100 μm (A, B, and E), 200 μm (C and F). CB, cortical bone; outside, area outside cortical bone. Spearman’s correlation coefficient test (D) and unpaired 2-tailed Student’s t test were used.