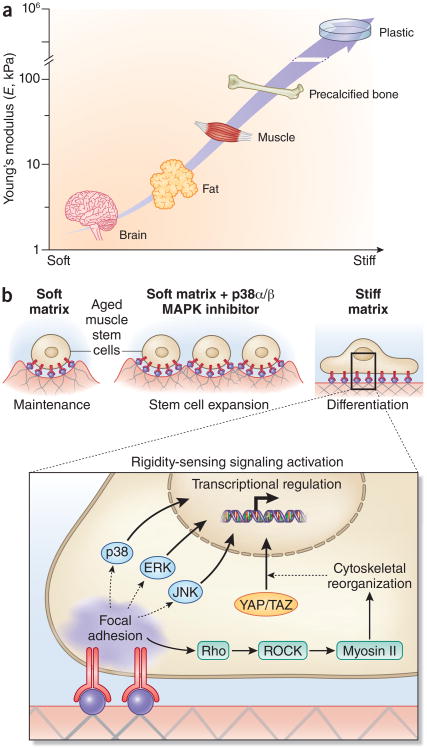
Figure 4.
Biophysical regulation of muscle stem cells in aging. (a) Tissue elasticity, the biophysical property of tissues and cell culture materials most commonly associated with effects on cell proliferation and differentiation, both in stem cells from skeletal muscle and other tissues, is typically characterized by a Young's modulus (E). The Young's modulus describes the elastic deformability of a material, and is proportional to the amount of force (stress) required to achieve a given deformation (strain). Characteristic tissue elasticities include brain and mammary glands (E = ∼1 kPa), adipose (∼5 kPa), skeletal muscle (∼10−30 kPa, and precalcified bone (∼50 kPa)102,113 Tissue culture substrates such as polystyrene and glass have typical Young's moduli of ∼106 kPa (ref. 114). In aging and muscular dystrophy, skeletal muscles become progressively fibrotic, resulting in increased rigidity92,93. (b) For muscle stem cells from aged mice, a soft 12-kPa matrix (mimicking the rigidity of healthy skeletal muscle31) supports muscle stem cell self-renewal expansion in synergy with targeted inhibition of the p38α/β MAPK signaling pathway29. A soft matrix alone supports aged MuSC maintenance whereas a stiff (106 kPa) matrix induces the differentiation of aged MuSCs. Cells sense the rigidity of their surrounding substrate through a variety of adhesion-dependent signaling pathways, which result in cellular deformations (for example, elongation) and transcriptional responses. Across many cell types, substrate rigidity is sensed by integrin clustering and focal adhesion stabilization, which yields prolonged activation of both canonica MAPK pathways (p38, ERK, JNK) and Rho/Rho-associated kinase (ROCK) signaling and subsequent myosin II–mediated cytoskeletal reorganization, leading to YAP/TAZ transcription factor translocalization115. Together, these and other adhesion-activated signaling pathways confer sensitivity to matrix stiffness in numerous cell types.