Abstract
The neural song circuit is enhanced in male compared to female zebra finches due to differential rates of incorporation and survival of cells between the sexes. Two double-label immunohistochemical experiments were conducted to increase the understanding of relationships between newly generated cells (marked with bromodeoxyuridine, BrdU) and those expressing brain derived neurotrophic factor (BDNF) and vimentin, a marker for radial glia. The song systems of males and females were investigated at post-hatching day 25 during a heightened period of sexual differentiation (following BrdU injections on days 6-10) and in adulthood (following a parallel injection paradigm). In both HVC (proper name) and the robust nucleus of the archopallium (RA), about half of the BrdU-positive cells expressed BDNF across sexes and ages. Less than 10% of the BDNF-positive cells expressed BrdU, but this percentage was greater in juveniles than adults. Across both brain regions, more BDNF-positive cells were detected in males compared to females. In RA, the number of these cells was also greater in juveniles than adults. In HVC, the average cross-sectional area covered by the vimentin labeling was greater in males than females and in juveniles compared to adults. In RA, more vimentin was detected in juveniles than adults, and within adults it was greater in females. In juveniles only, BrdU-positive cells appeared in contact with vimentin labeled fibers in HVC, RA and Area X. Collectively, the results are consistent with roles of BDNF and vimentin-labeled cells influencing sexual differentiated plasticity of the song circuit.
Keywords: sexual differentiation, songbird, radial glia, neurogenesis, AB_514483, AB_630940, AB_528504
INTRODUCTION
Newly generated cells migrate along radial glial processes to their destination. Radial glial cells function not only in this scaffolding capacity, but also as precursors of neurons and astroglia (information from mammalian systems: Hartfuss et al., 2003; Malatesta et al., 2000; Miyata et al., 2001; Noctor et al., 2001). While it was originally thought that new neurons are not generated in adulthood and that radial glial cells are no longer present after development, a growing body of knowledge across model systems no longer supports the idea that neurogenesis does not occur in adulthood (Alvarez-Buylla and Kirn, 1997; Opendak and Gould, 2015; Urban and Guillemot, 2014). In fact, adult neurogenesis is prevalent in fish, lizards and frogs, and is particularly well characterized in birds, including songbirds (Alvarez-Buylla and Kirn, 1997; D'Amico et al., 2011; Delgado-Gonzalez et al., 2011; Font et al., 2012; Schmidt et al., 2013).
Songbirds have provided an exceptional model for investigation of the incorporation of new cells in adulthood, as well as factors guiding neural development and function. Song is controlled by a well-defined set of neural circuits. Area X (in the striatum) and the lateral magnocellular nucleus of the anterior nidopallium (LMAN) regulate song learning and plasticity (Woolley and Kao, 2014; Woolley et al., 2014). The HVC (proper name) and robust nucleus of the arcopallium (RA) are cortical regions involving song production; HVC projects to RA, which in turn innervates motoneurons of the tracheosyringeal portion to control the muscles of syrinx (Aronov et al., 2008; McDonald and Kirn, 2012; Wade and Arnold, 2004). Most developmental research on songbirds has been conducted in zebra finches, in which only males sing. Parallel to the sex difference in singing behavior, HVC and RA become far larger in males than females over the course of development, and Area X is not visible in females using a variety of histological markers. HVC and Area X become sexually dimorphic largely through increased incorporation of cells. In contrast, increased cell death in females is primarily responsible for the development of the sex difference in the size of RA (reviewed in Wade and Arnold, 2004).
In songbirds, adult neurogenesis has primarily been studied in relation to seasonal changes in song syllables or stereotypy (Larson et al., 2014; Pytte et al., 2012; Walton et al., 2012). In canaries, neurons generated in adulthood migrate faster through regions with abundant radial glia processes (Alvarez-Buylla and Nottebohm, 1988) and are then functionally integrated in the brain (Paton and Nottebohm, 1984). More than half of the new neurons in adult HVC are replacements for RA projection neurons; they likely are important for recovery and maintenance of learned song (Alvarez-Buylla et al., 1990; reviewed in Brenowitz, 2015).
Proliferation and survival of neurons depends on a variety of neurotrophic factors, including brain derived neurotrophic factor (BDNF; McAllister et al., 1999; Sohrabji and Lewis, 2006). Several additional roles for this protein have been identified, including axon guidance, synapse formation, and learning and memory (Cohen-Cory et al., 2010; Cunha et al., 2010). In addition to roles during development, BDNF has also been implicated in the differentiation and maturation of neurons born in adulthood (Waterhouse et al., 2012; Waterhouse and Xu, 2009). BDNF supports the addition and/or survival of cells in the song system of birds, both during development and in adulthood (Brenowitz, 2013; Chen et al., 2013; Johnson et al., 1997; Louissaint et al., 2002). Up-regulation of BDNF expression in HVC during the sensorimotor integration period of song learning also facilitates the function of song acquisition (Dittrich et al., 2013).
We recently evaluated developmental changes in BDNF expression in zebra finches (Tang and Wade, 2013). By post-hatching day 25, males have a greater number of cells expressing BDNF protein than females in HVC. In RA, males have almost twice the number present in females, although the difference was not statistically significant with a Boneferroni correction. In both HVC and RA, small but significant increases were detected with age in the number of BDNF positive cells within males. In contrast, these cells decline with age in females, and by day 35 (for HVC) and adulthood (for RA) the borders of these brain regions can no longer be discerned from surrounding tissue with BDNF immunolabeling. The number of BDNF positive cells in Area X of males does not differ across ages, and Area X cannot be defined by BDNF labeling in females of any age. These data suggest that BDNF could play a role in the survival and/or incorporation of cells and the function of these brain regions.
We also collected data on the survival of cells in the song system born from post-hatching days 6-10 (Tang and Wade, 2009), as detected with bromodeoyxyuridine (BrdU). Labeling was quantified at day 25, a period of relatively rapid sexual differentiation across the song nuclei of interest. In HVC and RA, more BrdU positive cells were detected in males compared to females. Across HVC, RA and in the lateral striatum (which contains Area X), survival of these cells is increased in male compared to female zebra finches. More than half of the neurons in HVC are added after hatching, and many are added during this period to Area X as well. Neurogenesis is associated more with projection neurons in HVC and interneurons in Area X (Alvarez-Buylla and Kirn, 1997). Both of these brain regions also incorporate new neurons in adulthood, but RA does not as the majority of its neurons are born prior to hatching (Alvarez-Buylla and Kirn, 1997).
The present study was designed in part to address questions raised by our earlier work involving BDNF expression in adult females and the relationships between newly generated cells and BDNF. Specifically, in Experiment 1, we used a nissl stain to define the borders of HVC and RA, so that we could estimate total numbers of BDNF+ cells in adult females (these borders are not defined by BDNF labeling) and compare them to adult males and juveniles of both sexes. We also sought to determine whether cells within the song system that were born 2-3 weeks earlier express BDNF, and whether the proportions of these cells differ between the sexes and the ages. In Experiment 2 we evaluated vimentin, a well characterized marker for radial glia in songbirds, in an initial attempt at assessing whether this type of cell might play a role in the addition of new cells to song control regions. It is of particular interest to conduct these studies in zebra finches because they are opportunistic rather than seasonal breeders, and in parallel their songs and the general morphology of their song control nuclei remain stable in adulthood (Zann, 1996; Wade and Arnold, 2004). As in other species, new HVC-RA neurons are added in mature male zebra finches, but not as replacements for cells that die on a seasonal basis and without obvious consequences on song (Walton et al., 2012). Thus, effects of sex and age should suggest specific conclusions about roles of BDNF and radial glia in the process of masculinization.
MATERIALS AND METHODS
Animals
Zebra finches were raised in aviaries containing approximately 7 breeding pairs and their offspring. They were housed on a 12:12 light:dark cycle, and had seed and water available ad libitum. Each week, the birds also received spinach, oranges and bread mixed with hard-boiled chicken eggs. Nests were checked daily, and when a hatchling was found it was considered post-hatching day 1. Upon reaching adulthood (at least 100 days old) birds were moved to adjacent single-sex group cages. They could see and hear others in the colony without physically interacting with the opposite sex. All procedures were conducted in accordance with NIH guidelines and approved by the Michigan State University IACUC.
BrdU injections and tissue collection
Adults and post-hatching day 6 birds of both sexes received a daily injection of BrdU into the breast muscle for five consecutive days (in 0.75% saline, 50 μg/g body weight; Sigma #B5002, St. Louis, MO). Animals were euthanized 15 days after the last injection by rapid decapitation. Adults were sexed by their plumage, and the 25-day-old birds were sexed by examining their gonads. Brains were frozen in ice cold methylbutane and stored at −80°C, then sectioned at 20 μm and thaw-mounted onto SuperFrost Plus slides (Fisher Scientific, Hampton, NH). Six sets of slides containing coronal sections of each brain throughout the telecenphalon were stored with desiccant at −80°C until further processing. Sample sizes for all analyses are indicated with the results (Table 2 and figures).
Table 2.
Percentages (mean ± SEM) of BrdU and BDNF double labeled (DL) cells in the HVC and RA of zebra finches.
| 25-Day-Old | Adult | ||||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| HVC | DL/BrdU | 49.9±7.8 (5) | 59.9±7.0 (5) | 62.9±28.1 (6) | 45.8±30.8 (4) |
| DL/BDNF | 5.3±0.8 (5) | 7.8±1.1 (5) | 3.2±1.0 (6) | 1.9±1.5 (4) | |
| RA | DL/BrdU | 40.5±5.8 (7) | 49.8±10.7 (7) | 48.3±14.6 (5) | 50.0±35.4 (3)a |
| DL/BDNF | 7.0±1.5 (7) | 6.2±0.9 (7) | 2.4±1.0 (5) | 1.1±0.9 (5) | |
Numbers in parentheses represent sample sizes.
No BrdU was detected in 2 out of 5 birds, so the percentages of BrdU+ cells that co-expressed BDNF cannot be calculated for those birds. In one of the remaining three birds, no double-labeled cells were seen.
Experiment 1: Double-label immunohistochemistry for BrdU and BDNF
One set of slides from each animal was warmed to room temperature, rinsed in 0.1M phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde for 15 min, and then processed for BrdU immunolabeling as described previously (Tang and Wade, 2009) using a mouse monoclonal antibody (Table 1). The protein was visualized using an Elite ABC kit (Vector Labs, Burlingame CA) and the Vector SG blue/gray substrate, per manufacturer's instructions. Subsequently rinses in PBS and then incubated with BDNF antibody (Table 1) overnight at 4°C. Following biotin-SP-conjugated goat anti-rabbit IgG secondary antibody (1 μg/ml; 1 hr, Vector Labs) incubation and the ABC reaction, a diaminobenzidine chromogen was used with 0.0024% hydrogen peroxide to produce a brown reaction product.
Table 1.
Primary antibodies used for immunohistochemistry
| Name | Immunogen | Host | Antibody isotype | Manufacturer | Concentration |
|---|---|---|---|---|---|
| BrdU | Bromodeoxyuridine-bovine serum albumin conjugate BMC9318 - clone | Mouse | Monoclonal | Roche Applied Science Cat# 11170376001 Lot#: 1360172 RRID:AB_514483 |
1 μg/ml |
| BDNF | Human BDNF aa128-147 RHSDPARRGELSV-CDSISEW |
Rabbit | Polyclonal | Santa Cruz Biotechnology Cat# sc-546 Lot#: C1914 RRID:AB_630940 |
0.5 μg/ml |
| Vimentin | Homogenized adult canary brain | Mouse | Monoclonal | Developmental Studies Hybridoma Bank Cat# 40E-C Lot#:40E-C-c (6/21/12) RRID:AB 528504 |
1 μg/ml |
Experiment 2: Double-label immunohistochemistry for BrdU and Vimentin
An alternate set of sections from the same brains was processed as above for BrdU immunohistochemistry first, then rinsed with PBS and incubated with a vimentin antibody (Table 1) overnight at 4°C. Sections were then incubated in a biotin-SP-conjugated goat anti-mouse IgG secondary antibody (1 μg/ml; 1 hr, Vector Labs). The protein was visualized with Elite ABC reagents (Vector Labs) and diaminobenzidine with 0.0024% hydrogen peroxide.
Antibody Characterization
The BrdU primary antibody was validated using immunohistochemistry as described above on brain sections from juvenile zebra finches who either had (positive) or had not (negative control) received BrdU injections. The tissue from these birds was processed simultaneously, and labeling appeared only in the injected animal (data not shown). This antibody has been characterized in other species in the same way, including zebra fish (Lindsey et al., 2012). This study on fish also documented a lack of labeling with omission of the primary antibody. Characterization of the BDNF primary antibody on zebra finch tissues using western blot analysis and immunohistochemistry, including the use of a negative (preadsorption) control, is described in Tang and Wade (2012). Sequence of the immunizing peptide, as well as information on a preadsorption control and comparison to labeling of mRNA via in situ hybridization are reported for mouse by McClellan et al. (2010). The vimentin primary antibody was raised against homogenized canary brain to evaluate radial glia (Alvarez-Buylla and Nottebohm, 1988; Alvarez-Buylla et al., 1987), and has been used in many studies on zebra finch brains (Peterson et al., 2004; Saldanha et al., 2004; Spence et al., 2009; Wynne et al., 2008). The immunizing peptide is not available, so a preadsorption control could not be done. However, as in Alvarez-Buylla et al. (1987), a western blot analysis on protein extracted from zebra finch brain produced a single band at the expected ~50 kDa in our hands (data not shown).
Nissl staining
A third set of these sections was stained with cresyl violet. It was used to define the borders of HVC and RA for stereological estimates of BDNF labeled cells in Experiment 1. This was necessary because BDNF immunohistochemistry does not allow one to see edges of the brain regions in adult females (see above). It was also used for evaluation of the relative overall sizes of brain regions in Experiment 2 as a comparison to differences in vimentin labeling across groups (see below).
Experiment 1: Analysis of BrdU and BDNF labeled cells
HVC, RA and Area X were analyzed under brightfield illumination by an individual blind to the sex and age of the animals. The optical fractionator function of StereoInvestigator (Microbrightfield Inc., Williston, VT) was used to stereologically estimate the total number of labeled cells. In HVC and RA, analyses were conducted in both sexes. Area X was only evaluated in males as it is not present in females. The border of each brain region defined by nissl staining was traced, and this outline was transferred to the adjacent section containing BDNF immunohistochemical labeling. This process produced a reasonable approximation of the edges for analysis. While we could not trace the BDNF defined borders in adult females (see above), a comparison across the other three groups indicates only an average 6.1% difference in the volume of HVC (t12 = 1.17, p = 0.264) and a 10.3% difference in the volume of RA (t18 = 1.58, p=0.137) as defined by the two markers.
For all three brain regions, BDNF positive and double labeled cells were counted within 30 μm × 30 μm counting frames (as in Tang and Wade, 2013) on one side of the brain, randomly chosen. The number of BDNF labeled cells was generated by the software (with Gunderson coefficients ≤ 0.1). Because the numbers of BrdU expressing cells were quite low in adults, stereology could not be used to reliably generate estimated total numbers across the groups. Therefore, percentages of double labeled cells relative to BDNF and to BrdU were calculated based on the actual counts obtained, which include a subset of that total. Effects of age and sex were analyzed for each of these measures within HVC and RA by two-way ANOVA. In Area X, analyses were conducted within males between the ages by t-test (SPSS, version 21; IBM, Amonk, NY, USA).
Experiment 2: Analysis of BrdU and Vimentin
Vimentin alone was quantified by an individual blind to the experimental groups as follows. The HVC and RA from all animals and Area X from males were captured using Image J (National Institute of Health) under brightfield illumination in every section in which the region was visible on one side of the brain, randomly chosen. The border of each region was easy to see. It was traced and the ‘threshold’ function was used to the mark vimentin labeling in each region. The area covered by this immunohistochemical marker was generated by the software. These values were averaged across sections for each bird within the three brain regions. Effects of age and sex on the average cross-sectional area covered by vimentin labeling were analyzed by ANOVA (age × sex) for HVC and RA. A t-test was used to evaluate the effect of age within the male Area X. To confirm that statistically significant effects (see Results) were not simply due to differences in the sizes of the brain regions, the same statistics were computed on the average cross-sectional areas determined from nissl-stained sections.
Potential interactions between BrdU labeled cells and vimentin were qualitatively assessed by rating relative levels of BrdU positive cells appearing in direct contact with vimentin-positive fibers for each individual across the three brain regions (0 = no obvious contacts; 1= relatively few; 2 = moderate; 3 = substantial number of contacts). Examples of each of these scores are included in figures and identified in their captions.
Photographic Images
Photographs were taken using a Qimaging 2000R camera (sold by Microbrightfield, Inc.). Figures were compiled from these images in Adobe Photoshop. They were cropped and resized as necessary, and the brightness and color balance were adjusted to produce consistency within each figure.
RESULTS
Experiment 1: BrdU and BDNF
HVC
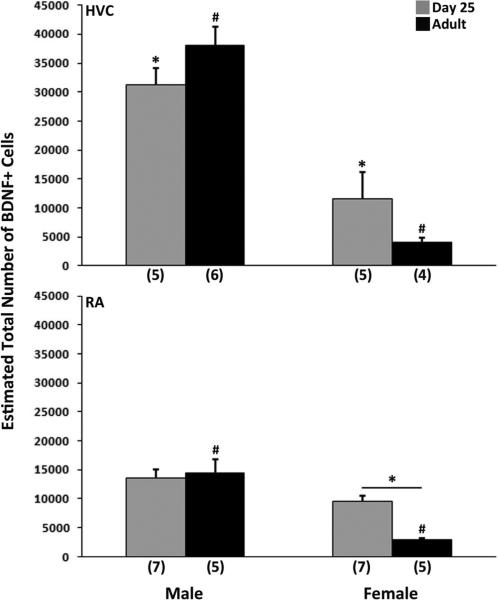
The estimated total number of BDNF positive cells was greater in males compared to females (F1,16 = 75.26, p < 0.001; Figures 1 and 2), and an age × sex interaction was detected (F1,16 = 5.29, p = 0.035), without a main effect of age (F1,16 = 0.01, p = 0.932). Two of the four pairwise comparisons were statistically significant. Within both ages, males had more BDNF expressing cells than females (both t8 > 4.01, p < 0.005; Bonferroni α = 0.0125); the interaction reflects a greater sex difference in adults than juveniles. Approximately half of the BrdU positive cells co-expressed BDNF, and this variable was not affected by sex or age (all F1,17 < 1.90, p > 0.187; Table 2). The proportion of BDNF labeled cells that co-expressed BrdU was far smaller (Table 2). While no effect of sex was detected (F1,17 = 0.34, p = 0.526), this percentage was significantly greater in juveniles compared to adults (F1,17 = 18.89, p < 0.001). The interaction between sex and age approached statistical significance (F1,17 = 4.46, p = 0.050). Pairwise comparisons indicated that effect of age was primarily due to data collected in females (t8 = 4.24, p = 0.003; α = 0.0125).
Figure 1.
Estimates of the total number of BDNF+ cells in the HVC (top) and RA (bottom) of 25-day-old and adult zebra finches. Means + SEM are indicated, and sample sizes are in parentheses. In HVC, a main effect of sex was detected, as was an interaction between age and sex. Males had more BDNF expressing cells than females at both ages, but the difference is larger in adults. In RA, a main effect of age and a significant effect of sex were detected. The two variables also interacted. For each brain region, common symbols over individual bars denote significant differences between them in pairwise comparisons.
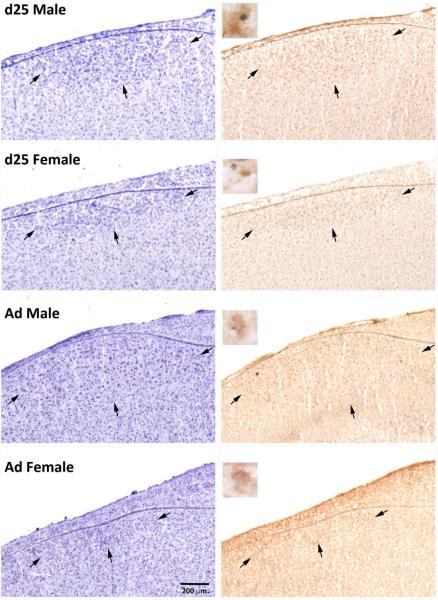
Figure 2.
Photographs of HVC in males and females at 25 days of age (d25) and in adulthood (Ad). The nissl stained sections (left) and those exposed to immunohistochemistry for BDNF and BrdU (right) are adjacent. Arrows indicate the borders of the brain region. Inserts (right column) show examples from the same animals of double-labeled cells in the two males, neighboring BrdU+ (blue/gray) and BDNF+ (brown) single labeled cells in the juvenile female, and a single labeled BDNF+ cell in the adult female.
RA
The number of BDNF positive cells was greater in males compared to females (F1,20 = 36.95, p < 0.001) and in juveniles compared to adults (F1,20 = 5.06, p = 0.036). An age × sex interaction was also detected (F1,20 = 8.59, p = 0.008; Figures 1 and 3). Two of the four pairwise comparisons were statistically significant. Within adults, males had more BDNF labeled cells than females (t8 = 5.65, p < 0.001; α = 0.0125), and within females, juveniles had more of these cells than adults (t1,8 = 6.02, p < 0.001). As in HVC, approximately 50% of the BrdU positive cells also expressed BDNF, and no effects of sex or age were detected (all F1,18 < 0.19, p > 0.665; Table 2). Similarly, the percentage of BDNF positive cells that co-expressed BrdU was quite low, but significantly greater in juveniles than adults (F1,19 = 24.21, p < 0.001). There was no effect of sex (F1,19 = 1.76, p = 0.200) or interaction between sex and age (F1,20 = 0.006, p = 0.939) on this measure.
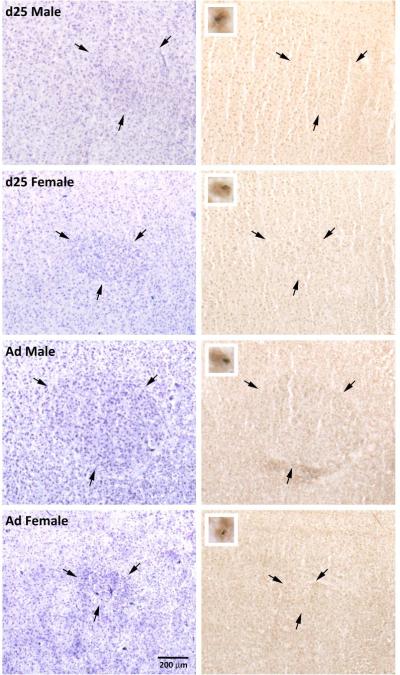
Figure 3.
Photographs of RA in males and females at 25 days of age (d25) and in adulthood (Ad). Pairs of nissl stained sections (left) and those exposed to immunohistochemistry for BDNF and BrdU (right) are adjacent. Arrows indicate the borders of the brain region. Inserts (right column) indicate cells labeled for both BrdU (blue/gray, nuclear) and BDNF (brown, cytoplasmic).
Area X
The estimated total number of BDNF positive cells was equivalent in juvenile and adult males (t12 = 0.052, p = 0.959; n = 7 per group; data not shown). It was not feasible to compare percentages of BrdU labeled cells co-expressing BDNF or vice versa across ages because no BrdU labeling was detected in five of the seven adult males.
Experiment 2a: BrdU and Vimentin
HVC
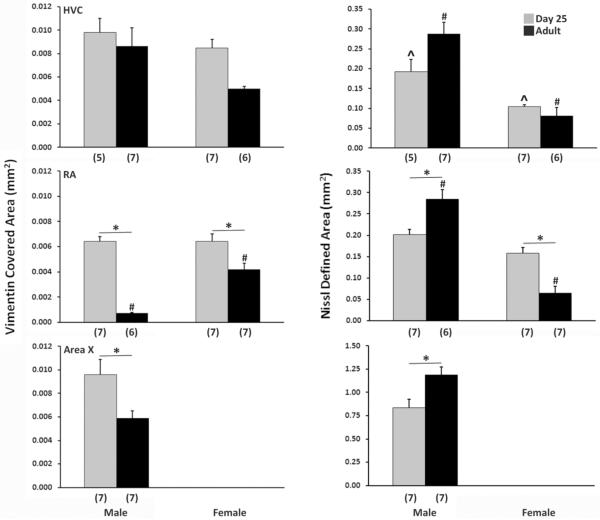
The average cross-sectional area labeled with vimentin was greater in males compared to females (F1,21 = 5.61, p = 0.028; Figure 4 left column, Figure 5). It was also significantly greater in juveniles compared to adults (F1,21 = 5.35, p = 0.031). While the interaction between sex and age was not statistically significant (F1,21 = 1.92, p = 0.287), the value in adult females was approximately half of that in the other three groups (Figure 4 left column).
Figure 4.
Average cross-sectional area covered by vimentin labeling (left) and of the full brain region defined by nissl staining (right) in the HVC (top), RA (middle) and Area X (bottom) of juvenile and adult zebra finches of both sexes. Means + SEM are indicated, and sample sizes are in parentheses. In HVC, main effects of both sex and age were detected for the area covered by vimentin labeling. A main effect of sex also existed for the overall cross-sectional area defined by nissl staining, and an interaction between sex and age was present. In RA, an increase in the vimentin covered area and a decrease in the nissl defined area were found in females compared to males. Significant effects of age for vimentin, and interactions of age and sex in both markers, were also detected. In Area X, juveniles had a larger area covered by vimentin and a smaller nissl defined area than adults. The brain region could not be defined in females. Asterisks spanning bars indicate effects of age within a sex; # denotes a sex difference within adults; ^ indicates a sex difference within juveniles.
Figure 5.
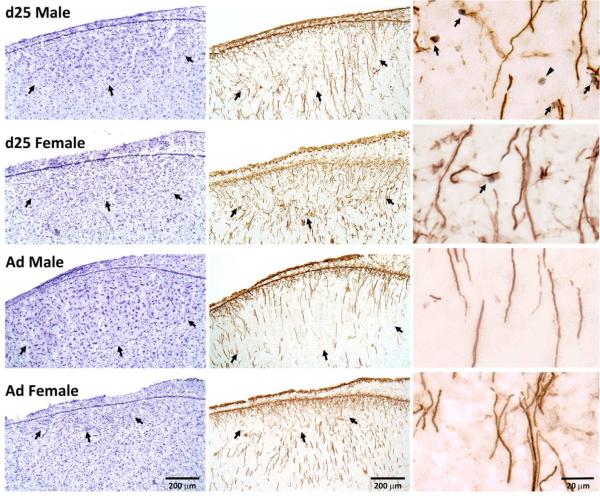
Photographs of HVC in males and females at 25 days of age (d25) and in adulthood (Ad). The nissl stained sections (left column) and those exposed to immunohistochemistry for vimentin and BrdU (middle column) are adjacent. Arrows in these sections indicate the borders of the brain region. The column on the right contains photos of higher magnification from near the center of HVC in the immunohistochemical sections immediately to their left. Arrows indicate BrdU+ nuclei and vimentin+ fibers that appear to be in contact, and the arrowhead in the top right image points to an isolated BrdU+ cell. Birds in the top two images (25-day-old) received scores of “2” in the qualitative assessment of contacts between BrdU− and vimentin-positive cells. The bottom two (adults) both received scores of “0”.
Among the 25-day-old birds, all males (5/5) and most females (6/7) had modest levels of BrdU positive cells that appeared in direct contact with vimentin labeling (mean ratings of 1.5 for males and 1.2 for females on the 0-3 scale; Figure 5 right column). Neighboring BrdU+ cells and vimentin+ processes were not detected in the HVC of any adult bird (0/7 males, 0/6 females).
RA
The area of RA covered by vimentin labeling exhibited significant main effects of sex (F1,22 = 14.16, p < 0.001; Figures 4 and 6) and age (F1,22 = 76.60, p < 0.001), as well as a significant interaction between the two variables (F1,22 = 14.89, p < 0.001). The quantity of labeling was greater in juveniles than adults for both males (t11 = 10.81, p < 0.001; α = 0.0125) and females (t11 = 3.01, p = 0.012). However, this age difference was far greater in males; very little labeling was detected in the RA of adult males (Figure 4 left column, Figure 6). Among adults, the average area covered by vimentin labeling was greater in females (t10 = 6.74, p < 0.001), whereas no sex difference on this measure was detected at post-hatching day 25 (t12 = 0.06, p = 0.953).
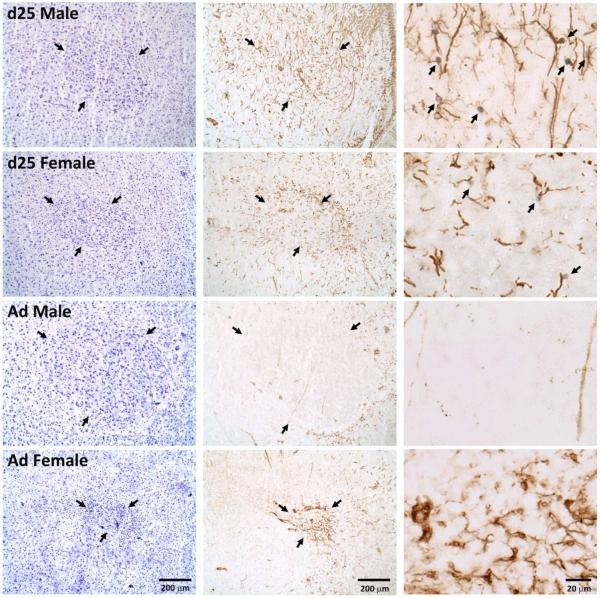
Figure 6.
Photographs of RA in males and females at 25 days of age (d25) and in adulthood (Ad). The nissl stained sections (left column) and those exposed to immunohistochemistry for vimentin and BrdU (middle column) are adjacent. Arrows in these sections indicate the borders of the brain region. The column on the right contains photos of higher magnification from near the center of RA in the immunohistochemical sections immediately to their left. Arrows indicate BrdU+ nuclei and vimentin+ fibers that appear to be in contact. Birds in the top two images (25-day-old) received scores of “3” in the qualitative assessment of contacts between BrdU− and vimentin-positive cells. The bottom two (adults) both received scores of “0”.
All juvenile males (7/7) and females (7/7) appeared to have close contacts between BrdU+ cells and vimentin+ processes. These levels were generally higher in RA (2.3 on the 0-3 scale; Figure 6) than in HVC (1.4 on the 0-3 scale; Figure 5) . No such contacts were detected in adults of either sex (0/6 males, 0/6 females).
Area X
Consistent with results from other markers (see above), Area X could not be defined by vimentin labeling in females, so the analysis in this brain region was conducted only for males. The average cross-sectional area covered by vimentin labeling was significantly greater in 25-day-old compared to adult males (t12 = 2.68, p = 0.020; Figure 4 left column, Figure 7).
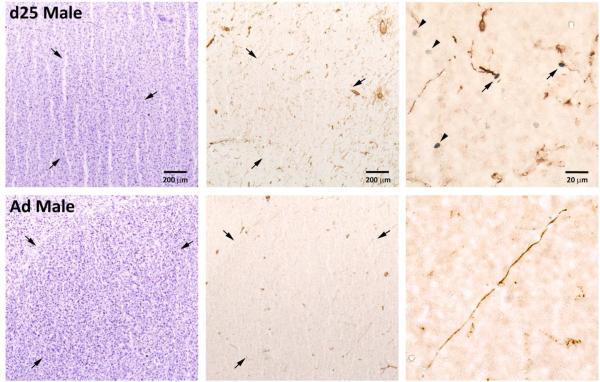
Figure 7.
Photographs of Area X in a 25-day-old (d25) and an adult (Ad) male. The nissl stained sections (left column) and those exposed to immunohistochemistry for vimentin and BrdU (middle column) are adjacent. Arrows in these sections indicate the borders of the brain region. The column on the right contains photos of higher magnification from near the center of Area X in the immunohistochemical sections immediately to their left. Arrows indicate BrdU+ nuclei and vimentin+ fibers that appear to be in contact, and the arrowheads in the top right image points to isolated BrdU+ cells. The bird in the top image (25-day-old) received a score of “1” in the qualitative assessmemt of contacts between BrdU− and vimentin-positive cells. The bottom individual (adult) received a “0”.
As indicated above, BrdU was not detected in the Area X of most adult males, although vimentin labeling was apparent in all males (Figure 7). Even in the adult males that did express some BrdU, none of these labeled cells were neighboring vimentin-positive processes. Apparent contacts between these cell types were also quite limited in juvenile males (5 out of 7) in Area X at the lowest level (1 on the 0-3 scale).
Experiment 2b: General morphological differences due to sex and age
The average cross-sectional areas of both HVC (F1,21 = 46.50, p < 0.001) and RA (F1,22 = 69.54, p < 0.001) as identified in nissl-stained sections were far greater in males than females (Figure 4 right column). Main effects of age were not detected in either brain region (both F1,22 ≤ 2.67, p ≥ 0.117), but significant interactions were present in both HVC (F1,21 = 7.54, p = 0.012) and RA (F1,22 = 39.23, p < 0.001). In HVC, the sex difference was present at both ages, but was larger in adults (t11 = 5.82, p < 0.001) than juveniles (t10 = 3.85, p = 0.003). In RA, three of the pairwise comparisons were statistically significant. The average cross-sectional area of this brain region was greater in adult males than adult females (t11 = 8.84, p < 0.001; α = 0.0125). This value was also larger in adult males compared to juvenile males (t1,11 = 3.85, p = 0.003) and smaller in adult females compared to juvenile females (t1,12 = 4.82, p < 0.001). Finally, in Area X, the brain region was larger overall in adult compared to 25-day-old males whereas significantly less vimentin was detected in adults compared to the juveniles (t12 = 3.08, p = 0.010).
DISCUSSION
The present data add new information on the mechanisms likely to regulate neural plasticity of the forebrain song circuit. Co-expression of BrdU and BDNF suggests the neurotrophin may support the survival and incorporation of newly generated cells in the zebra finch song circuit, not only during development but also in adulthood. In addition, the pattern of vimentin expression between sexes and ages along with its relationship with BrdU positive cells provides new insights into how radial glial cells may contribute to structural change in the song system.
BDNF and the incorporation of new cells
New cells are added to the brains of songbirds both during development and in adulthood. In young animals, incorporation and survival of these cells is critical for learning and production of song in males (reviewed in Alvarez-Buylla and Kirn, 1997); the role in females is less clear. In adults, studies of neurogenesis have largely been associated with seasonal changes in the songs of canaries (Alvarez-Buylla, 1990; Alvarez-Buylla, 1992; Alvarez-Buylla and Kirn, 1997; Alvarez-Buylla et al., 1994; Doupe, 1994; Goldman, 1998; Vellema et al., 2010). However, adult neurogenesis also occurs in zebra finches, in which males establish stable songs that typically do not change once they become mature (review in Alvarez-Buylla and Kirn, 1997). Across songbirds, BDNF has been implicated in the addition of new cells to the song system at both life stages (Brenowitz, 2013; Brenowitz, 2015; Chen et al., 2013; Louissaint et al., 2002).
The present work expands our knowledge of BDNF by comparing its relationship to newly generated cells in the song system between the sexes and across developmental stages. The percentage of BDNF labeled cells that co-expressed BrdU in HVC was greater in juveniles than adults, primarily due to significant decrease in adult females. Juveniles also had a higher percentage of BDNF positive cells that co-expressed BrdU in RA compared to adults, although the effect of age could not be assessed in Area X. Overall, few BrdU labeled cells were detected in adults, particularly in Area X. The data across developmental stages are consistent with those of DeWulf and Bottjer (2002) who documented enhanced cell division in juvenile compared to adult brains, which correlated with neuron addition to HVC and Area X of developing males.
The relatively low level of BrdU we detected in adults compared to juveniles likely diminished the chances of detecting co-expression with BDNF. However, the data may be biologically meaningful. They suggest that BDNF expressing cells are more likely to have recently generated in juveniles compared to adults. Combined with the information that about half of the surviving new born cells at both ages express BDNF, these data are consistent with the idea that BDNF facilitates the survival and/or incorporation of new cells into the forebrain motor pathway regulating song production. Further work specifically testing this mechanism should be done in developing zebra finches, with particular attention to sexual differentiation.
The sex difference in BDNF immunoreactive cells in the present study is consistent with our earlier work (Tang and Wade, 2012; Tang and Wade, 2013). More cells express this protein in males than females in HVC. The effects of age detected in the present study also parallel our previous studies. In HVC, the estimated total number of BDNF positive cells was very similar to our developmental study (Tang and Wade, 2013), and as in the earlier work a significant increase was detected between post-hatching day 25 and adulthood in males. In our previous experiment, BDNF in the HVC of females was not quantified after day 25 because the border was not visible with this immunohistochemical marker. The present study using nissl defined borders allowed us to document a reduction in the number of BDNF labeled cells in females between day 25 and adulthood by more than 50%, although this difference did not quite reach statistical significance. These results parallel sex and age differences in the number of neurons in HVC (Kirn and DeVoogd, 1989). Thus, it is possible that in the HVC of both sexes changes in BDNF expressing cells simply follow changes in cell number, or it is possible that BDNF facilitates the incorporation and/or survival of cells. BDNF manipulation during zebra finch brain development is required to distinguish between these possibilities.
Data from adult songbirds is consistent with an active role of BDNF in the regulation of neural structure and function. For example, infusion of BDNF into the HVC of adult male canaries 2-3 weeks after neurons are born in the spring extends their life expectancy (Alvarez-Borda et al., 2004). Similarly, BDNF treatment in the HVC of adult female canaries triples the number of new neurons. Infusion of an antibody to BDNF blocks the increase of new neurons in testosterone-treated females (Rasika et al., 1999). In development, upregulation of BDNF-mRNA specifically in the HVC of juvenile male zebra finches increases copying of song syllables from a tutor (Dittrich et al., 2013). Additionally, treating cultures of juvenile female Bengalese finch brains with BDNF for 24 hours increases the density of BrdU positive cells along the ventricular zone overlaying HVC, and this increase can be blocked with a BDNF antibody (Chen et al., 2014).
The absolute numbers and patterns between the present and our previous studies are also similar for RA. In our earlier work (Tang and Wade, 2013), males exhibited an increase in BDNF labeled cells from post-hatching day 25 to 65, but we could not test for a main effect of sex into adulthood due to lack of data from adult females. A decrease of BDNF positive cells in females from post-hatching days 25 to 65 is consistent with the present study, after which the border couldn't be seen in adult females which made accurate counts impossible. Using a nissl defined RA border, the current work indicates that the sex difference is also present in adults. In males, the present result is similar to the data we collected earlier in which no difference exists in the number of BDNF positive cells between 25-day-old juveniles and adults.
Unlike HVC, the previous data from males (days 25 to 65) on the number of cells expressing BDNF in RA does not simply parallel age-related changes in neuron number, as this value remains relatively stable from day 25 through at least day 60 (Kirn and DeVoogd, 1989). Therefore, it seems likely that additional existing cells begin to express BDNF as the animals mature, which could facilitate their survival. Additionally, the number of pyknotic cells in RA increases dramatically in juvenile females from day 20 to 30 while cell numbers decline overall between post-hatching days 20 and 60, suggesting that many BDNF cells die in the female RA during development (Kirn and DeVoogd, 1989). Thus, in females loss of cells in general could account for changes in the number of cells expressing BDNF, or lack of BDNF could be responsible for the cell loss. These ideas need to be directly tested in future studies.
Data from juvenile zebra finches and adult white-crowned sparrows are consistent with BDNF affecting the structure of RA. Specifically, in developing zebra finches, BDNF prevents the death of deafferented RA neurons (Johnson et al., 1997). In the sparrows, BDNF mRNA levels increase in HVC in response to breeding conditions, BDNF infusion into RA promotes breeding-like changes in soma size and neuronal density, and blocking BDNF activity in RA inhibits the response to breeding conditions (Wissman and Brenowitz, 2009).
Results from our previous (Tang and Wade, 2013) and the current study indicate no change in the number of BDNF labeled cells in Area X from day 25 to adulthood in males. In contrast, neuron number increases in Area X during this period (Nordeen and Nordeen, 1988), suggesting a more limited role of BDNF in the incorporation or survival of cells in this song nucleus compared to the others, at least across the range of ages we investigated.
Potential roles for glial cells
The present work is consistent with a function for the processes of radial glia in guiding the migration of newly generated cells into functional circuits. Substantial expression of vimentin exists in the brains of juvenile male and female zebra finches, and BrdU positive cells were detected immediately adjacent to these fibers in the vast majority of juveniles. This close apposition occurs only in juveniles, and consistently in both males and females where quantified. Thus, the data suggest a potentially greater role for radial glial fibers facilitating the migration of newly generated cells into the song control nuclei of juveniles compared to adults. This interpretation may be particularly valid for RA, in which the quantity of vimentin detected in adult males was by far the lowest of the three groups investigated, despite the cross-sectional area of the brain region being the largest (Figure 4).
While the general pattern of close contacts between BrdU-positive cells and vimentin-labeled processes does not provide direct evidence for a role of radial glial cells in sexual differentiation of the song circuit, earlier work from our lab in concert with the current data suggest that possibility. That is, stereological estimates of cells generated from days 6-10 and incorporated into HVC and RA by day 25 reveal a significant increase in males compared to females in both brain regions (Tang and Wade, 2009). About 17% of these cells are neurons in HVC and 12% in RA, across both sexes. Thus, radial glial cells likely guide both neurons and other types of cells into the motor pathway for song production, and they probably do so to a greater extent in juvenile males than females.
Our data are also consistent with those from cultures of developing Bengalese finch brains, in which the density of newly generated cells along vimentin immunoreactive fibers in HVC is greater in developing males than females (Chen et al., 2014). This sex difference exists in neurons, which comprised about 15% of the BrdU-labeled cells in that study. However, it is possible that the diffence exists in glial cells as well. It is important to note that in our current work we do not know the phenotype of the newly generated cells in either sex or at either age. Future experiments should determine whether they are neurons or glia.
Effects of sex and age on the area covered by vimentin labeling are largely incongruent with the average cross-sectional area of the brain regions (determined in nissl stained tissue). These differences suggest that this protein plays an active role in modulating brain structure, and that the quantity detected does not passively follow the size of these regions. Data on volumes of the brain regions suggest largely same idea. That is, the volume of HVC (Nixdorf-Bergweiler, 1996) increases in males from days 10-60, and then appears steady until adulthood. In females, the volume declines from days 10-40 and then is stable until adulthood. The decrease in vimentin detected in females from day 25 to adulthood in the present study parallels this decline in the overall size of the brain region. However, the lack of change in vimentin in males does not parallel the overall volume. Similarly, the approximate 15% increase in vimentin labeling in 25-day-old males compared to females of the same age is far less than the roughly 80% increase in the volume of HVC (Nixdorf-Bergweiler, 1996). In RA, there is no sex difference in vimentin labeling at day 25, but a sex difference in the volume of the region does exist (reviewed in Wade and Arnold, 2004). In the RA of adults, a female-biased sex difference was detected in vimentin labeling, wherease the cross-sectional area (Figure 4) and the volume are much larger in males than females (Konishi and Akutagawa, 1985; Konishi and Akutagawa, 1990; Wade and Arnold, 2004). Finally, the volume of Area X in males doubles from day 25 to adulthood, yet the vimentin change is large and in the opposite direction.
Collectively the data raise questions regarding the role of vimentin in adults, particularly in the RA of adult females where the quantity is greater than that of adult males, despite a greatly reduced overall size of the brain region. The morphology of these immunoreactive vimentin cells might provide a clue about function. In HVC and Area X, the vimentin labeling across all birds is primarily in elongated processes, which is consistent with radial glia. In RA, the morphology of cells labeled with vimentin seems to change across ages in a sexually dimorphic manner. It is generally in relatively long processes in males, and substantially greater in quantity in juveniles compared to adults. In contrast, the processes seem qualitatively shorter in females, and the shape of the labeled cells seems to differ in adults (Figure 6). In mature females, the vimentin immunoreactivity includes both cell bodies and more elaborate processes. The morphology of these cells is consistent with astrocytes (Holtman et al., 2015; Kamphuis et al., 2015; Komitova et al., 2006; Mamber et al., 2012; Sofroniew, 2009; von Bohlen und Halbach, 2011; Wynne et al., 2008) or possibly microglia (Alonso, 2005; Garcia-Ovejero et al., 2013, Streit et al., 1988), both of which express vimentin. It also appears similar to cells in Bengalese finches labeled with glial fibrillary acidic protein (GFAP; Chen et al., 2014). Thus, as RA decreases in size with age in females, less vimentin labeling is detected overall. However, vimentin labeled cells appear more densly packed (see Figure 6), and the phenotype of the cells may differ between adults and juveniles. This idea warrants further investigation. Both astrocytes and microglia appear to play key roles in sexual differentiation of the structure of the rodent brain, in ways that have implications for adult reproductive behavior (Lenz and McCarthy, 2015; McCarthy et al., 2003).
Summary and future directions
The present studies provide new insights into the potential for BDNF to facilitate the incorporation and/or survival of newly generated cells in the song system. They also document that, similar to adult songbirds and other species, vimentin positive radial glial processes are positioned to facilitate migration of these new cells during development. This relationship is generally diminished in adulthood in both RA and Area X, potentially due to lower levels of cell genesis, but possibly also because other mechanisms may be important for cell addition, including local birth. It will be critical now to conduct studies that directly test the function of BDNF across the sexes and ages, as well as to determine the phenotype of vimentin-positive cells, in zebra finches. Particularly in the adult female RA, these cells may include astrocytes and/or microglia and their function should be evaluated. In addition, it would be valuable to conduct similar studies across sexes and ages in other songbirds. It can be difficult to breed them in captivity, but comparing results between zebra finches and canaries (which unlike zebra finches breed seasonally, modify their songs in adulthood, and have females that sing a bit) should provide exciting new information about specific mechanisms.
ACKNOWLEDGMENTS
We thank Levi Storks and Rebecca Benjamin for their assistance, and Linda Beach for comments on the manuscript.
Grant sponsor: NIH R01:MH096705
Footnotes
ROLE OF AUTHORS
All authors had full access to the data in the study and take responsibility for its integrity and accuracy. Study concept and design: YPT and JW; acquisition of data: YPT; analysis and interpretation of data: YPT and JW; manuscript preparation: YPT and JW.
CONFLICT OF INTEREST STATEMENT
We have no conflicts of interest.
New cells in the song systems of juvenile and adult zebra finches express brain derived neurotrophic factor (BDNF), and more BDNF+ cells are present in males than females. These new cells appear in contact with vimentin-labeled fibers in juveniles only, suggesting that BDNF and radial glia may influence sexual differentiation.
LITERATURE CITED
- Alonso G. NG2 proteoglycan-expressing cells of the adult rat brain: possible involvement in the formation of glial scar astrocytes following stab wound. Glia. 2005;49(3):318–338. doi: 10.1002/glia.20121. [DOI] [PubMed] [Google Scholar]
- Alvarez-Borda B, Haripal B, Nottebohm F. Timing of brain-derived neurotrophic factor exposure affects life expectancy of new neurons. Proc Natl Acad Sci USA. 2004;101(11):3957–3961. doi: 10.1073/pnas.0308118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A. Mechanism of neurogenesis in adult avian brain. Experientia. 1990;46(9):948–955. doi: 10.1007/BF01939388. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A. Neurogenesis and plasticity in the CNS of adult birds. Exp Neurol. 1992;115(1):110–114. doi: 10.1016/0014-4886(92)90232-f. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Buskirk DR, Nottebohm F. Monoclonal antibody reveals radial glia in adult avian brain. J Comp Neurol. 1987;264(2):159–170. doi: 10.1002/cne.902640203. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kirn JR. Birth, migration, incorporation, and death of vocal control neurons in adult songbirds. J Neurol. 1997;33(5):585–601. [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kirn JR, Nottebohm F. Birth of projection neurons in adult avian brain may be related to perceptual or motor learning. Science. 1990;249(4975):1444–1446. doi: 10.1126/science.1698312. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Ling CY, Yu WS. Contribution of neurons born during embryonic, juvenile, and adult life to the brain of adult canaries: regional specificity and delayed birth of neurons in the song-control nuclei. J Comp Neurol. 1994;347(2):233–248. doi: 10.1002/cne.903470207. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Nottebohm F. Migration of young neurons in adult avian brain. Nature. 1988;335(6188):353–354. doi: 10.1038/335353a0. [DOI] [PubMed] [Google Scholar]
- Aronov D, Andalman AS, Fee MS. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science. 2008;320(5876):630–634. doi: 10.1126/science.1155140. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA. Testosterone and brain-derived neurotrophic factor interactions in the avian song control system. Neuroscience. 2013;239:115–123. doi: 10.1016/j.neuroscience.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz EA. Transsynaptic trophic effects of steroid hormones in an avian model of adult brain plasticity. Front Neuroendocrinol. 2015;37:119–28. doi: 10.1016/j.yfrne.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Zhang X, Zhao Y, Zhou X, Sun L, Zeng S, Zuo M, Zhang X. Sexual differences in cell proliferation in the ventricular zone, cell migration and differentiation in the HVC of juvenile Bengalese finch. PLoS One. 2014;9(5):e97403. doi: 10.1371/journal.pone.0097403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Ye R, Goldman SA. Testosterone modulation of angiogenesis and neurogenesis in the adult songbird brain. Neuroscience. 2013;239:139–48. doi: 10.1016/j.neuroscience.2012.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol. 2010;70(5):271–288. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Front Mol Neurosci. 2010;3:1. doi: 10.3389/neuro.02.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico LA, Boujard D, Coumailleau P. Proliferation, migration and differentiation in juvenile and adult Xenopus laevis brains. Brain Res. 2011;1405:31–48. doi: 10.1016/j.brainres.2011.06.032. [DOI] [PubMed] [Google Scholar]
- Delgado-Gonzalez FJ, Gonzalez-Granero S, Trujillo-Trujillo CM, Garcia-Verdugo JM, Damas-Hernandez MC. Study of adult neurogenesis in the Gallotia galloti lizard during different seasons. Brain Res. 2011;1390:50–58. doi: 10.1016/j.brainres.2011.03.027. [DOI] [PubMed] [Google Scholar]
- DeWulf V, Bottjer SW. Age and sex differences in mitotic activity within the zebra finch telencephalon. J Neurosci. 2002;22(10):4080–4094. doi: 10.1523/JNEUROSCI.22-10-04080.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich F, Ter Maat A, Jansen RF, Pieneman A, Hertel M, Frankl-Vilches C, Gahr M. Maximized song learning of juvenile male zebra finches following BDNF expression in the HVC. Eur J Neurosci. 2013;38(9):3338–3344. doi: 10.1111/ejn.12329. [DOI] [PubMed] [Google Scholar]
- Doupe AJ. Songbirds and adult neurogenesis: a new role for hormones. Proc Natl Acad Sci USA. 1994;91(17):7836–7838. doi: 10.1073/pnas.91.17.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Font E, Barbosa D, Sampedro C, Carazo P. Social behavior, chemical communication, and adult neurogenesis: studies of scent mark function in Podarcis wall lizards. Gen Comp Endocrinol. 2012;177(1):9–17. doi: 10.1016/j.ygcen.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Garcia-Ovejero D, Arevalo-Martin A, Paniagua-Torija B, Sierra-Palomares Y, Molina-Holgado E. A cell population that strongly expresses the CB1 cannabinoid receptor in the ependyma of the rat spinal cord. J Comp Neurol. 2013;521(1):233–251. doi: 10.1002/cne.23184. [DOI] [PubMed] [Google Scholar]
- Goldman SA. Adult neurogenesis: from canaries to the clinic. J Neurobiol. 1998;36(2):267–286. [PubMed] [Google Scholar]
- Hartfuss E, Forster E, Bock HH, Hack MA, Leprince P, Luque JM, Herz J, Frotscher M, Gotz M. Reelin signaling directly affects radial glia morphology and biochemical maturation. Development. 2003;130(19):4597–4609. doi: 10.1242/dev.00654. [DOI] [PubMed] [Google Scholar]
- Holtman IR, Raj DD, Miller JA, Schaafsma W, Yin Z, Brouwer N, Wes PD, Moller T, Orre M, Kamphuis W, Hol EM, Boddeke EW, Eggen BJ. Induction of a common microglia gene expression signature by aging and neurodegenerative conditions: a co-expression meta-analysis. Acta Neuropathol Comm. 2015;3(1):31. doi: 10.1186/s40478-015-0203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F, Hohmann SE, DiStefano PS, Bottjer SW. Neurotrophins suppress apoptosis induced by deafferentation of an avian motor-cortical region. J Neurosci. 1997;17(6):2101–2111. doi: 10.1523/JNEUROSCI.17-06-02101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis W, Kooijman L, Orre M, Stassen O, Pekny M, Hol EM. GFAP and vimentin deficiency alters gene expression in astrocytes and microglia in wild-type mice and changes the transcriptional response of reactive glia in mouse model for Alzheimer's disease. Glia. 2015;63(6):1036–1056. doi: 10.1002/glia.22800. [DOI] [PubMed] [Google Scholar]
- Kirn JR, DeVoogd TJ. Genesis and death of vocal control neurons during sexual differentiation in the zebra finch. J Neurosci. 1989;9(9):3176–3187. doi: 10.1523/JNEUROSCI.09-09-03176.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitova M, Perfilieva E, Mattsson B, Eriksson PS, Johansson BB. Enriched environment after focal cortical ischemia enhances the generation of astroglia and NG2 positive polydendrocytes in adult rat neocortex. Exp Neurol. 2006;199(1):113–121. doi: 10.1016/j.expneurol.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Konishi M, Akutagawa E. Neuronal growth, atrophy and death in a sexually dimorphic song nucleus in the zebra finch brain. Nature. 1985;315(6015):145–147. doi: 10.1038/315145a0. [DOI] [PubMed] [Google Scholar]
- Konishi M, Akutagawa E. Growth and atrophy of neurons labeled at their birth in a song nucleus of the zebra finch. Proc Natl Acad Sci USA. 1990;87(9):3538–3541. doi: 10.1073/pnas.87.9.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson TA, Thatra NM, Lee BH, Brenowitz EA. Reactive neurogenesis in response to naturally occurring apoptosis in an adult brain. J Neurosci. 2014;34(39):13066–13076. doi: 10.1523/JNEUROSCI.3316-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, McCarthy MM. A starring role for microglia in brain sex differences. Neuroscientist. 2015;21(3):306–321. doi: 10.1177/1073858414536468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey BW, Darabie A, Tropepe V. The cellular composition of neurogenic periventricular zones in the adult zebrafish forebrain. J Comp Neurol. 2012;520:2275–2316. doi: 10.1002/cne.23065. [DOI] [PubMed] [Google Scholar]
- Louissaint A, Jr., Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34(6):945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127(24):5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- Mamber C, Kamphuis W, Haring NL, Peprah N, Middeldorp J, Hol EM. GFAPdelta expression in glia of the developmental and adolescent mouse brain. PLoS One. 2012;7(12):e52659. doi: 10.1371/journal.pone.0052659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Todd BJ, Amateau SK. Estradiol modulation of astrocytes and the establishment of sex differences in the brain. Ann NY Acad Sci. 2003;1007:283–297. doi: 10.1196/annals.1286.027. [DOI] [PubMed] [Google Scholar]
- McClellan KM, Stratton MS, Tobet SA. Roles for γ-aminobutyric acid in the development of the paraventricular nucleus of the hypothalamus. J Comp Neurol. 2010;518:2710–2728. doi: 10.1002/cne.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald KS, Kirn JR. Anatomical plasticity in the adult zebra finch song system. J Comp Neurol. 2012;520(16):3673–3686. doi: 10.1002/cne.23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31(5):727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Nixdorf-Bergweiler BE. Divergent and parallel development in volume sizes of telencephalic song nuclei in male and female zebra finches. J Neurol. 1996;375(3):445–456. doi: 10.1002/(SICI)1096-9861(19961118)375:3<445::AID-CNE7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409(6821):714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW. Sex and regional differences in the incorporation of neurons born during song learning in zebra finches. J Neurosci. 1988;8(8):2869–2874. doi: 10.1523/JNEUROSCI.08-08-02869.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opendak M, Gould E. Adult neurogenesis: a substrate for experience-dependent change. Trends Cogn Sci. 2015;19(3):151–161. doi: 10.1016/j.tics.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Paton JA, Nottebohm FN. Neurons generated in the adult brain are recruited into functional circuits. Science. 1984;225(4666):1046–1048. doi: 10.1126/science.6474166. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Lee DW, Fernando G, Schlinger BA. Radial glia express aromatase in the injured zebra finch brain. J Comp Neurol. 2004;475(2):261–269. doi: 10.1002/cne.20157. [DOI] [PubMed] [Google Scholar]
- Pytte CL, George S, Korman S, David E, Bogdan D, Kirn JR. Adult neurogenesis is associated with the maintenance of a stereotyped, learned motor behavior. J Neurosci. 2012;32(20):7052–7057. doi: 10.1523/JNEUROSCI.5385-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasika S, Alvarez-Buylla A, Nottebohm F. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22(1):53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Schlinger BA, Micevych PE, Horvath TL. Presynaptic N-methyl-D-aspartate receptor expression is increased by estrogen in an aromatase-rich area of the songbird hippocampus. J Comp Neurol. 2004;469(4):522–534. doi: 10.1002/cne.11035. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Strahle U, Scholpp S. Neurogenesis in zebrafish - from embryo to adult. Neural Dev. 2013;8:3. doi: 10.1186/1749-8104-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32(12):638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Lewis DK. Estrogen-BDNF interactions: implications for neurodegenerative diseases. Front Neuroendocrinol. 2006;27(4):404–414. doi: 10.1016/j.yfrne.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence RD, Zhen Y, White S, Schlinger BA, Day LB. Recovery of motor and cognitive function after cerebellar lesions in a songbird: role of estrogens. The Eur J Neurosci. 2009;29(6):1225–1234. doi: 10.1111/j.1460-9568.2009.06685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Graeber MB, Kreutzberg GW. Functional plasticity of microglia: a review. Glia. 1988;1(5):301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- Tang YP, Wade J. Effects of estradiol on incorporation of new cells in the developing zebra finch song system: potential relationship to expression of ribosomal proteins L17 and L37. Dev Neurobiol. 2009;69(7):462–475. doi: 10.1002/dneu.20721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Wade J. 17β-Estradiol regulates the sexually dimorphic expression of BDNF and TrkB proteins in the song system of juvenile zebra finches. PLoS One. 2012;7(8):e43687. doi: 10.1371/journal.pone.0043687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Wade J. Developmental changes in BDNF protein in the song control nuclei of zebra finches. Neuroscience. 2013;250:578–587. doi: 10.1016/j.neuroscience.2013.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban N, Guillemot F. Neurogenesis in the embryonic and adult brain: same regulators, different roles. Front Cell Neurosci. 2014;8:396. doi: 10.3389/fncel.2014.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellema M, van der Linden A, Gahr M. Area-specific migration and recruitment of new neurons in the adult songbird brain. J Comp Neurol. 2010;518(9):1442–1459. doi: 10.1002/cne.22281. [DOI] [PubMed] [Google Scholar]
- von Bohlen, Halbach O. Immunohistological markers for proliferative events, gliogenesis, and neurogenesis within the adult hippocampus. Cell Tissue Res. 2011;345(1):1–19. doi: 10.1007/s00441-011-1196-4. [DOI] [PubMed] [Google Scholar]
- Wade J, Arnold AP. Sexual differentiation of the zebra finch song system. Ann NY Acad Sci. 2004;1016:540–559. doi: 10.1196/annals.1298.015. [DOI] [PubMed] [Google Scholar]
- Walton C, Pariser E, Nottebohm F. The zebra finch paradox: song is little changed, but number of neurons doubles. J Neurosci. 2012;32(3):761–774. doi: 10.1523/JNEUROSCI.3434-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse EG, An JJ, Orefice LL, Baydyuk M, Liao GY, Zheng K, Lu B, Xu B. BDNF promotes differentiation and maturation of adult-born neurons through GABAergic transmission. J Neurosci. 2012;32(41):14318–14330. doi: 10.1523/JNEUROSCI.0709-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse EG, Xu B. New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol Cell Neurosci. 2009;42(2):81–89. doi: 10.1016/j.mcn.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissman AM, Brenowitz EA. The role of neurotrophins in the seasonal-like growth of the avian song control system. J Neurosci. 2009;29(20):6461–6471. doi: 10.1523/JNEUROSCI.0638-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SC, Kao MH. Variability in action: Contributions of a songbird cortical-basal ganglia circuit to vocal motor learning and control. Neuroscience. 2014;296:39–47. doi: 10.1016/j.neuroscience.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Woolley SC, Rajan R, Joshua M, Doupe AJ. Emergence of context-dependent variability across a basal ganglia network. Neuron. 2014;82(1):208–223. doi: 10.1016/j.neuron.2014.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne RD, Walters BJ, Bailey DJ, Saldanha CJ. Inhibition of injury-induced glial aromatase reveals a wave of secondary degeneration in the songbird brain. Glia. 2008;56(1):97–105. doi: 10.1002/glia.20594. [DOI] [PubMed] [Google Scholar]
- Zann R. The zebra finch: Synthesis of field and laboratory studies. Oxford University Press; New York: 1996. [Google Scholar]