Abstract
Although best managed by surgical resection, we present a case of Listeria monocytogenes endovascular graft infection alternatively treated with graft retention and antibiotic induction followed by a lifelong suppressive course. The epidemiological, pathological, and clinical features of this unique entity are reviewed.
Keywords: aneurysm, doxycycline, endovascular graft, Listeria monocytogenes
Endovascular graft infection with Listeria monocytogenes is quite rare despite the pathogen's predilection for bacteremia [1, 2]. Endovascular infections with L monocytogenes may be smoldering in onset thus causing diagnostic delay [3]. However, once diagnosed, the best chance of cure necessitates prolonged antimicrobial therapy and graft removal. We describe an illustrative case in which the endovascular graft was preserved and an induction course of antibiotics was followed by lifelong suppression without recurrence of infection.
CASE PRESENTATION
A 68-year-old woman with idiopathic cardiomyopathy, atrial fibrillation with Maze III procedure, and thoracic aortic aneurysm with endovascular repair 2 years before presentation was referred for 3 months of antecedent outpatient symptoms. Before symptom onset, she had spent time on a rural farm where she consumed unpasteurized dairy and developed 1 week of nonbloody diarrhea. Stool studies were not performed. One month before presentation she had generalized malaise and a nonsustained subjective fever. She was seen by her primary care physician, and blood cultures were obtained that grew Gram-positive rods in 1 of 2 bottles from each of 2 peripheral venipunctures. She was admitted to the hospital where a repeat venous blood culture also grew Gram-positive rods in 1 of 2 bottles. All cultured growth was ultimately identified as L monocytogenes by the Vitek System (BioMérieux, France) with susceptibility to penicillin (minimum inhibitory concentration 1.0 µg/mL), vancomycin (≤1.0 µg/mL), rifampin (≤0.5 µg/mL), gentamicin (≤1.0 µg/mL), ciprofloxacin (≤1.0 µg/mL), trimethoprim/sulfamethoxazole (≤0.5 µg/mL), and doxycycline (≤4.0 µg/mL), and presumed resistance to azithromycin (>1.0 µg/mL) performed at Mayo Laboratories. The patient's other laboratory parameters were remarkable for a hemoglobin of 10.7 g/dL and an erythrocyte sedimentation rate of 27 mm/hour.
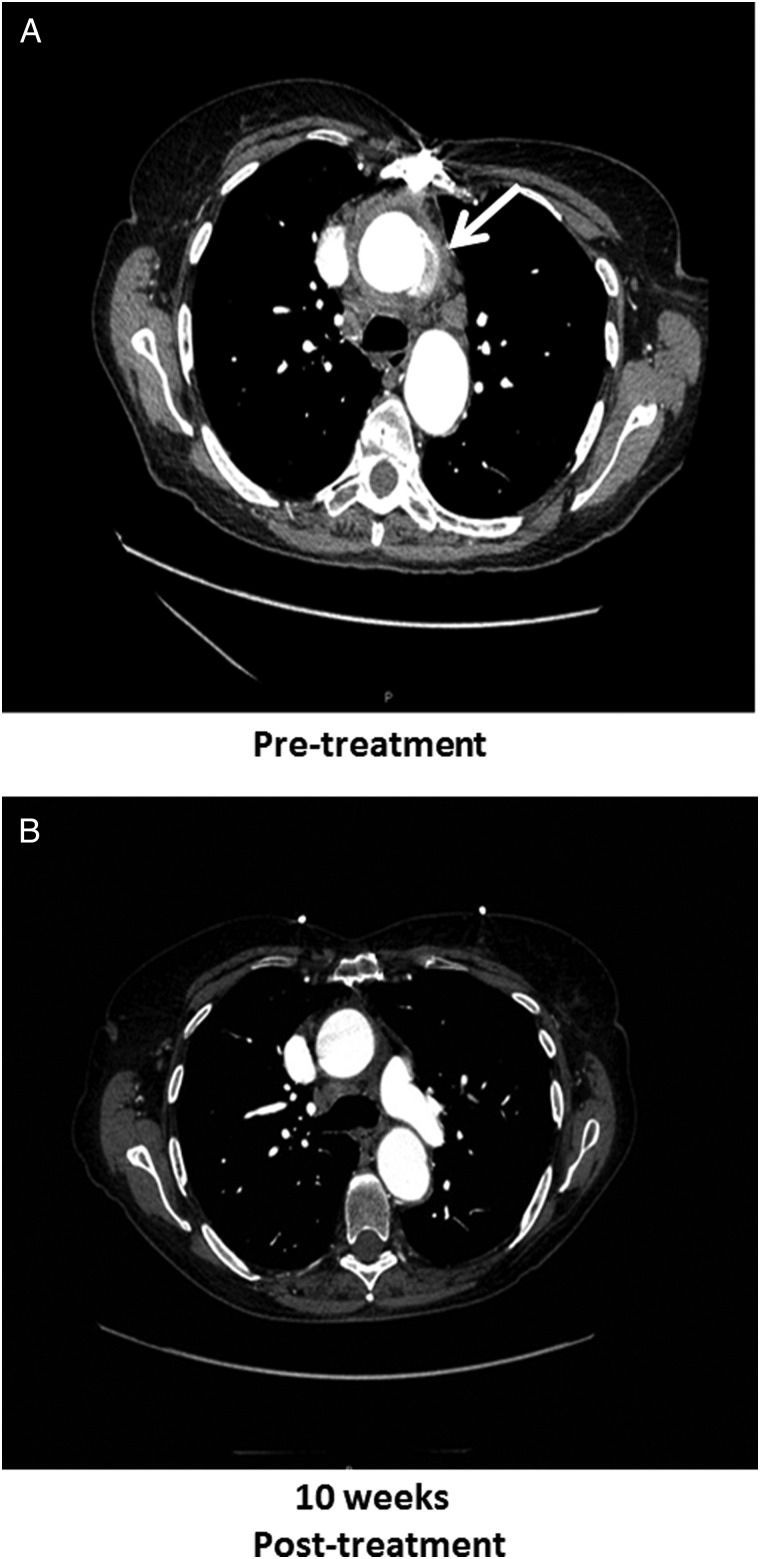
Given concern for prior gastroenteritis, consequent bacteremia, and then endovascular seeding, a computed tomography (CT) angiogram of the chest was performed revealing new thoracic aortic perigraft inflammation from root to arch with subcarinal and paratracheal lymphadenopathy consistent with infection (Figure 1). Because graft removal was deemed too high of risk, a cautious trial of antimicrobial therapy alone was initiated. Ampicillin and synergistic gentamicin were administered until acute kidney injury forced discontinuation of the gentamicin and presumed angioedema compelled ampicillin discontinuation after only 2 days of administration. She completed 6 weeks of intravenous vancomycin at 1 gram (15 mg/kg) every 12 hours. After intravenous therapy, intolerance to ciprofloxacin and trimethoprim/sulfamethoxazole ultimately led to lifelong suppression with doxycycline at 100 mg (1.5 mg/kg) twice daily. A follow-up CT angiogram after 10 weeks of antimicrobial therapy revealed resolution of periaortic inflammation (Figure 1), and she remains without recurrence of infection, including normal erythrocyte sedimentation rate and C-reactive protein, more than 3 years after diagnosis.
Figure 1.
The graphic illustrates initial computed tomography (CT) angiography of the chest (A) demonstrating ascending thoracic aortic repair and new perigraft inflammation (arrow) from root to arch with associated lymphadenopathy. A follow-up CT angiography (B) with resolution of inflammation is also shown.
DISCUSSION
Despite persistent outbreaks of listeriosis with bacteremia as a common consequence, we report 1 of the few cited cases of endovascular graft infection. The Centers for Disease Control and Prevention estimate that 1600 illnesses and 260 deaths due to listeriosis occur annually in the United States [4], yet as few as 18 cases of primary aneurysmal infection with L monocytogenes have been reported (2 of the thoracic aorta), with only 7 cases of endovascular graft infection (1 of the thoracic aorta) [1–3, 5, 6]. Similar to the case presented and unlike most other adult cases of listeriosis, immunosuppression and hematologic malignancy do not appear to be predisposing factors for endovascular graft infection.
The duration from infection to invasive endovascular disease is variable with a mean of 30 days reported and is likely due to the bacteria's remarkable ability to infect epithelial cells, escape phagosomes, divide using host machinery, push to the cell surface to form a filapod for ingestion by an adjacent cell, and thereby escape neutrophil, antibody, or complement [7–9]. Despite the increased risk for endovascular graft infections early in the perioperative period before purported “endothelialization,” our case and the majority in the literature presented more than 6 months after graft placement [3].
CONCLUSIONS
Although controlled trials do not exist to guide management, endovascular graft infections with L monocytogenes are likely best managed by graft resection in combination with long-term antimicrobial therapy with ampicillin or sulfonamides. If ampicillin is used, then gentamicin can be given at synergistic doses. Rifampin is effective in vitro, but when given in combination it is not superior to ampicillin alone [8]. Mortality remains high in cases of L monocytogenes endovascular graft infections. The review of reported cases through 1998 described death in 41% of patients, including all who did not undergo surgical resection. However, more recently, graft retention with successful outcome has been described. A case of abdominal aortic endograft preservation was treated with perigraft catheter drainage, intravenous amoxicillin/clavulanic acid (unspecified dose and duration), and ultimately 6 weeks of trimethoprim/sulfamethoxazole (800 mg/160 mg) 3 times daily, and the patient remained cured after ∼1 year [6]. Another patient with thoracic aortic endograft infection was treated with 21 days of ampicillin but the infection recurred, and given the complexity of the proposed repair the patient underwent another 6 weeks of ampicillin (2 grams every 6 hours), initially with 4 overlapping weeks with gentamicin, followed by amoxicillin (1 gram every 8 hours) for 8 weeks, and at 1 year the patient remained asymptomatic [3]. It is possible that other recent examples of graft retention and poor outcome were simply not reported in literature. Hence, in the absence of compelling evidence to the contrary, in cases such as ours where the graft is preserved, we would favor a lifelong suppressive course of a tolerable oral antibiotic with the narrowest of spectrum.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Heikkinen L, Valtonen M, Lepantalo M et al. . Infrarenal endoluminal bifurcated stent graft infected with Listeria monocytogenes. J Vascul Surg 1999; 29:554–6. [DOI] [PubMed] [Google Scholar]
- 2.Gauto AR, Cone LA, Woodard DR et al. . Arterial infections due to Listeria monocytogenes: report of four cases and review of world literature. Clin Infect Dis 1992; 14:23–8. [DOI] [PubMed] [Google Scholar]
- 3.Rohde H, Horstkotte MA, Loeper S et al. . Recurrent Listeria monocytogenes aortic graft infection: confirmation of relapse by molecular subtyping. Diagn Microbiol Infect Dis 2004; 48:63–7. [DOI] [PubMed] [Google Scholar]
- 4.Scallan E, Hoekstra RM, Angulo FJ et al. . Foodborne illness acquired in the United States--major pathogens. Emerg Infect Dis 2011; 17:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paccalin M, Amoura Z, Brocheriou I et al. . [Infectious aneurysm due to Listeria monocytogenes: a new case and review of the literature]. Rev Med Interne 1998; 19:661–5. [DOI] [PubMed] [Google Scholar]
- 6.Saleem BR, Berger P, Zeebregts CJ et al. . Periaortic endograft infection due to Listeria monocytogenes treated with graft preservation. J Vasc Surg 2008; 47:635–7. [DOI] [PubMed] [Google Scholar]
- 7.Lorber B. Listeria monocytogenes In: Mandell GL, Bennett JE, Dolin R, ed. Principles and Practice of Infectious Diseases, 7th ed Philadelphia, PA: Churchill, Livingstone, Elsevier; 2010: pp 2707–14. [Google Scholar]
- 8.Lorber B. Listeriosis. Clin Infect Dis 1997; 24:1–11. [DOI] [PubMed] [Google Scholar]
- 9.Wing EJ, Gregory SH. Listeria monocytogenes: clinical and experimental update. J Infect Dis 2002; 185:18–24. [DOI] [PubMed] [Google Scholar]