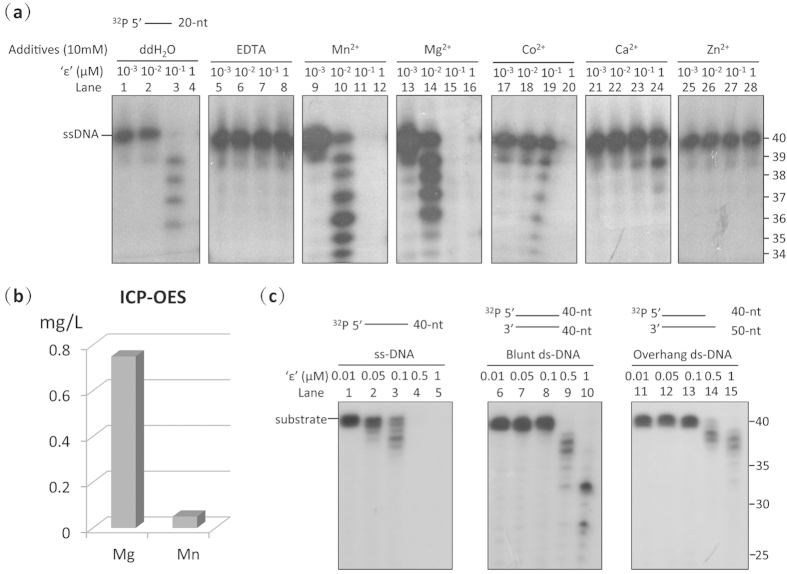
Figure 2. The ‘ε’ subunit of Mtb DNA pol III has exonuclease activity.
(a) Ion dependency of the exonuclease activity of the ‘ε’ subunit. The ‘ε’ subunit is a 3′–5′ exonuclease which is activated by Mg2+ and Mn2+, and inhibited by EDTA, Co2+, Ca2+and Zn2+. (b) ICP-OES quantitation of metal ions in the purified ‘ε’ subunit. The native ‘ε’ subunit binds significantly more Mg2+ (~0.75 mg/L) than Mn2+ (less than 0.05 mg/L), and is thus a Mg2+-dependent exonuclease. The mean of three replicate samples analysed is shown. (c) The exonuclease activity of the ‘ε’ subunit shows a preference for ssDNA over blunt-dsDNA or 5′ overhanging-dsDNA as substrate. Substrate types are shown above each panel. Results presented are representative of at least three replicate experiments.