Figure 5. Investigation of the functional interactions between the subunits of the αβ2‘ε’ complex of Mtb DNA pol III.
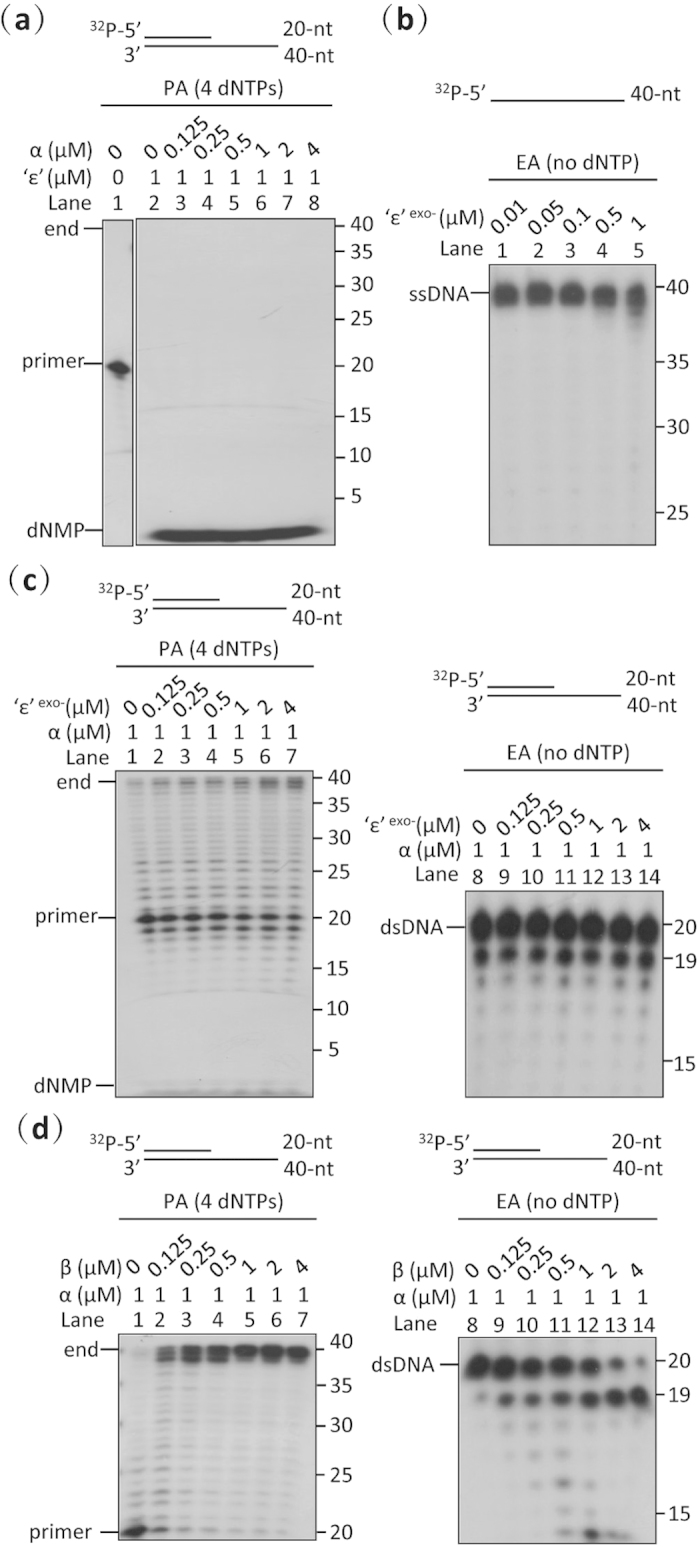
(a) Primer-extension assay of different ratios of α and ‘ε’ subunits using a primed dsDNA substrate. Mixtures of α and ‘ε’ subunits exhibit unexpectedly high exonuclease activity on paired duplex DNA and no polymerase activity in vitro. Increasing amounts of the α subunit fail to weaken the exonuclease activity of the ‘ε’ subunit in vitro. (b) Exonuclease assay of the ‘ε’ mutant (‘ε’exo-) using a ssDNA substrate. Mutation of the active site of the ‘ε’ subunit (D20A, E22A and D104A) successfully destroys its exonuclease activity. (c) Primer-extension and exonuclease assays of the α subunit with increasing amounts of ‘ε’-exo using a primed dsDNA substrate. The ‘ε’-exo mutant modestly enhances the polymerase activity of the α subunit (left panel), but has no effect on its exonuclease activity (right panel). (d) Primer-extension and exonuclease assays of the α subunit with increasing amounts of β2 clamp using a primed dsDNA substrate. While the polymerase activity of the α subunit is strongly promoted by the presence of the β2 clamp (left panel), its exonuclease activity is only slightly increased (right panel). The reaction conditions of the polymerase (PA) and exonuclease assays (EA) were the same, except that the 4 dNTPs were omitted in the exonuclease assay. The primed dsDNA substrate was produced by annealing a 5’-32P-labeled ssDNA primer (20-mer) to an unlabeled ssDNA template (40-mer). Results presented are representative of at least three replicate experiments.
