Figure 6. Role of the β2 clamp in the αβ2‘ε’ complex of Mtb DNA pol III.
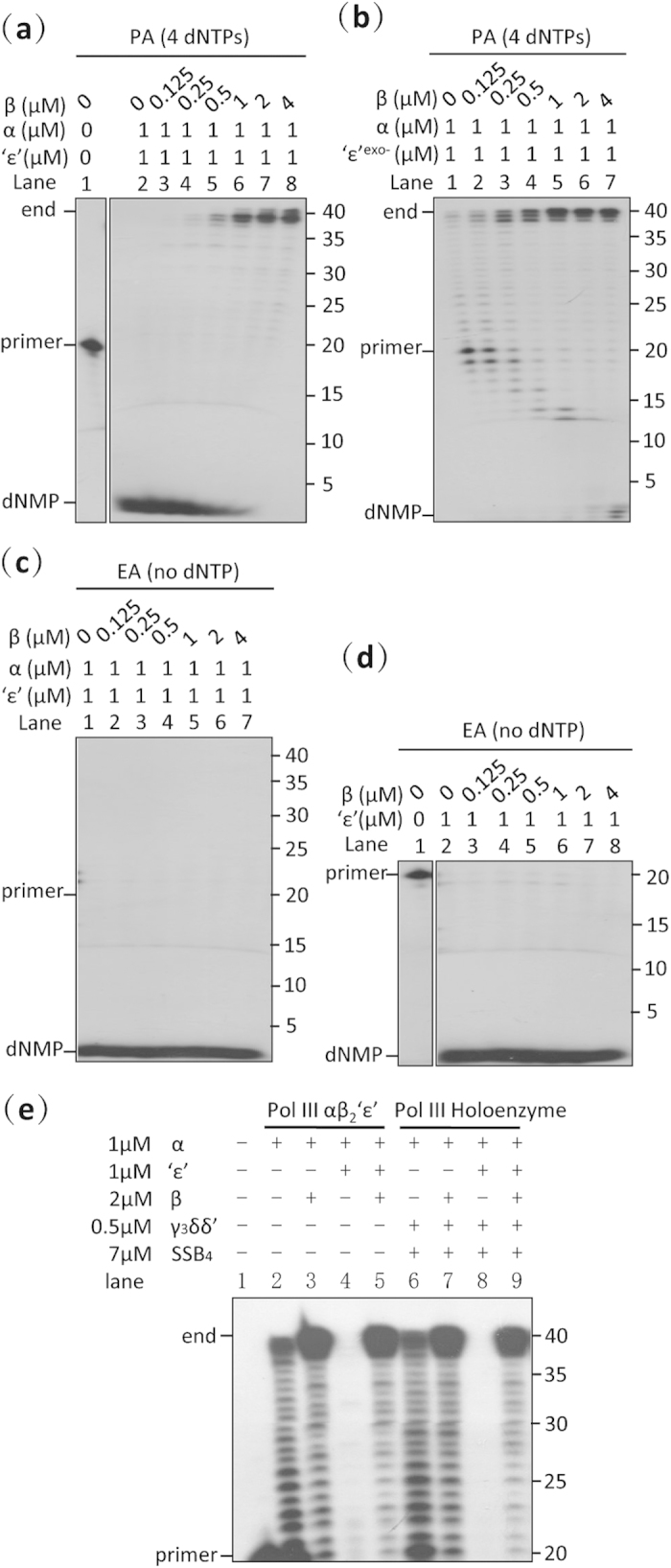
(a) Primer-extension assay of the αβ2‘ε’ replicase. The β2 clamp strongly promotes the polymerization and reduces the exonuclease activity of the αβ2‘ε’ complex. (b) Primer-extension assay of the αβ2‘ε’exo- replicase. Once the exonuclease activity of the ‘ε’ subunit is mutated, the β2 clamp promotes the polymerase activity, and very slightly enhances the exonuclease activity of the αβ2‘ε’exo- replicase. (c) Exonuclease assays of the αβ2‘ε’ replicase. Increasing the amount of the β2 clamp does not reduce the exonuclease activity of the αβ2‘ε’ complex in the absence of the 4 dNTPs. (d) Exonuclease assays of different ratios of ‘ε’ and the β2 clamp. Increasing the amount of the β2 clamp fails to directly preclude the exonuclease activity of the ‘ε’ subunit in vitro. (e) Primer-extension assay of Mtb DNA pol III subassemblies. Equimolar mixtures of the α and ‘ε’ subunits exhibited only exonuclease activity, both in the presence (holoenzyme) and absence (αβ2‘ε’) of the clamp loader and SSB, and the presence of the β2 clamp reduced exonuclease activity and restored polymerase activity. The reaction conditions of the polymerase (PA) and exonuclease assays (EA) were the same, except that the 4 dNTPs were omitted in the exonuclease assay. The primed dsDNA substrate used in all the above experiments was produced by annealing a 5′-32P-labeled ssDNA primer (20-mer) to an unlabeled ssDNA template (40-mer). Results presented are representative of at least three replicate experiments.
