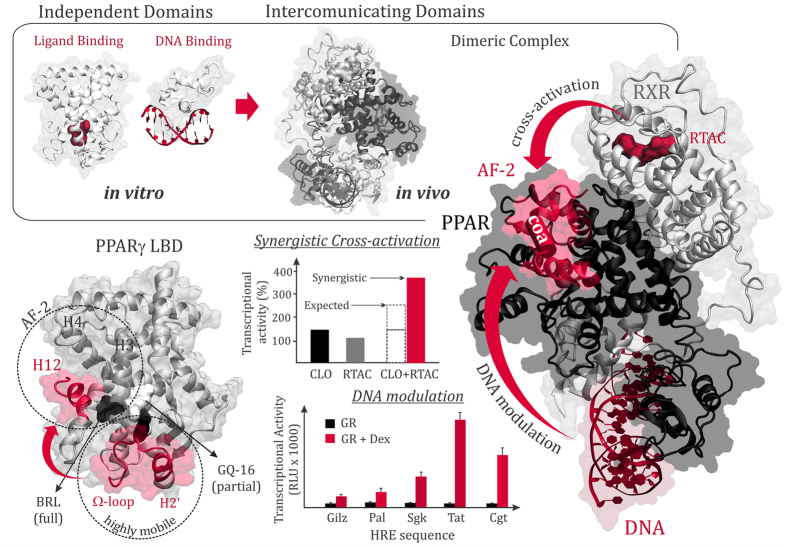
Figure 1. Allosteric communications in the PPARγ-RXR α nuclear receptor (NR).
NRs are modular proteins formed by ligand (LBD) and DNA (DBD) binding domains that display intrinsic activity in vitro. In vivo, they form dimeric complexes, such as the PPARγ-RXRα complex, that bind to DNA and show allosteric interdomains communication (top and right panels). RXR ligands, such as 9-cis-retinoic acid (RTAC), can cross-activate PPAR by inducing conformational changes in its activation function-2 (AF-2) region, comprising the helix 12 (H12) in the LBD. Synergistic cross-activation is known to take place when PPAR is bound to one of its own bona fide ligands, such as clofibric acid (CLO, illustrative histograms reproduced from ref. 23). As observed for other NRs (such as glucocorticoid, estrogen and vitamin D receptors), transcriptional activity can be modulated by the DNA sequence (illustrative histograms reproduced from ref. 21), suggesting allosteric communications between the DNA and the AF-2 region. The PPARγ LBD (bottom left panel) differs from other NRs by having an extra helix (H2’) followed by an extremely large and flexible Ω-loop. Full agonists, such as rosiglitazone (BRL), activate the AF-2 region by forming a direct interaction with the H12, while partial agonists (such as GQ-16) do not make direct contact with H12, indicating alternative allosteric mechanisms that may involve the Ω-loop and H2’ flexible regions.