Figure 1.
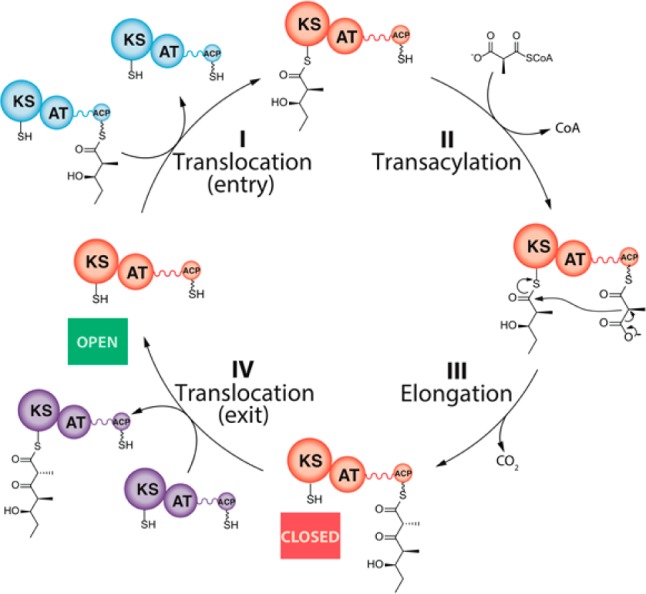
Core catalytic cycle for a representative PKS module: A typical module (orange) catalyzes at least four transformations: (I) translocation of an incoming polyketide chain from the ACP of the upstream module (blue) to the active site cysteine residue of the KS domain; (II) AT-catalyzed transacylation of a substituted malonyl chain extender (depicted as a methylmalonyl unit) from the corresponding CoA thioester to the pantetheinyl side chain of an ACP; (III) chain elongation via a decarboxylative condensation between the acyl-KS and methylmalonyl-ACP; and (IV) translocation of the newly elongated chain to the KS domain of the downstream module (purple). In addition to these four reactions, most PKS modules harbor one or more auxiliary enzymatic domains that catalyze additional chain modification reactions (ketoreduction, dehydration, enoyl reduction) between steps III and IV. Although PKS modules are homodimeric, for convenience they are depicted as monomers in this and other schemes in this report. Their homodimeric architecture does not affect the conclusions drawn from this study.
