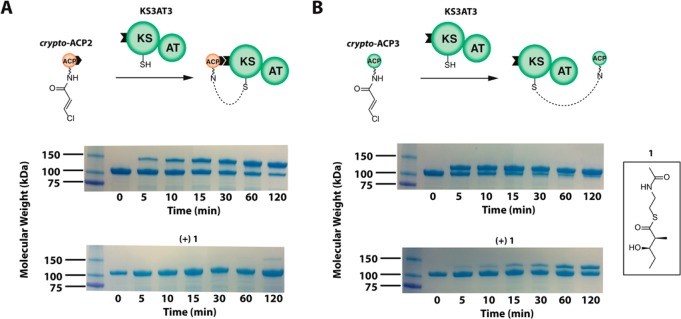
Figure 2.
Recognition of the catalytic KS-AT core of a PKS module by its two partner ACP domains. Transient protein–protein interactions were detected by cross-linking the catalytic core of DEBS module 3 (composed of functional KS and AT domains as well as flanking linkers, most notably the docking domains depicted as a black tab and explained in Figure S1) to modified forms of each of its two ACP partners, chain donor ACP2 and chain acceptor ACP3, both harboring an electrophilic probe at the end of their respective pantetheinyl arms. These modified proteins, designated crypto-ACPs, are excellent probes of protein–protein interactions between ACP domains and their partner enzymes in fatty acid and polyketide synthases.17,18 Synthesis of crypto-ACP2 and crypto-ACP3 is described in the Supporting Information. (A) crypto-ACP2 (250 μM; with its C-terminal flanking peptide, as explained in Figure S1) was incubated with KS3AT3 (50 μM) in the absence (top) and presence (bottom) of 5 mM diketide 1, which competitively acylates the KS active site. The lower band corresponds to the KS3AT3 protein (100 kDa), while the upper band corresponds to the cross-linked adduct between the crypto-ACP and KS3AT3 (121 kDa). (B) crypto-ACP3 (250 μM) was incubated with KS3AT3 (50 μM) in the absence (top) and presence (bottom) of 5 mM diketide 1. The smaller 12-kDa mass difference between monomeric KS3AT3 and the cross-linked adduct (112 kDa) reflects the absence of a C-terminal docking domain on crypto-ACP3.