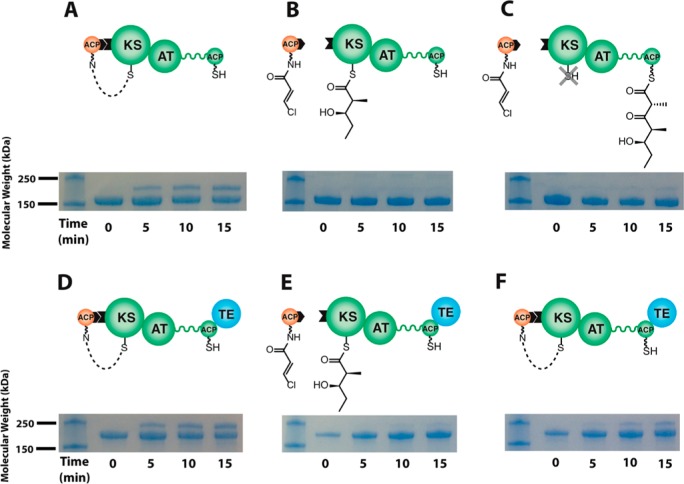
Figure 3.
Recognition of a PKS module by its upstream ACP partner. Protein interactions were detected by cross-linking either DEBS module 3 (A–C) or module 3 + TE (D–F) to a modified form of its upstream ACP partner from DEBS module 2. Synthesis of crypto-ACP2 is described in the Supporting Information. crypto-ACP2 (250 μM) was incubated with module 3 or module 3 + TE (50 μM) in the absence (A, D) or presence (B, E) of 10 mM diketide 1 (shown in Figure 2), which acylates the KS active site of module 3 or module 3 + TE. The lower protein band in each SDS–PAGE image corresponds to module 3 (160 kDa) or module 3 + TE (186 kDa), whereas the upper band corresponds to the cross-linked adduct between the crypto-ACP and module 3 (181 kDa) or module 3 + TE (207 kDa). In panels C and F, the crypto-ACP (250 μM) was incubated with module 3 or module 3 + TE (50 μM) that had been preincubated with diketide 1 plus methylmalonyl-CoA thus enabling elongation of the diketide chain. Chain elongation was initiated by addition of methylmalonyl-CoA, and allowed to proceed for 1 min with either module. Whereas the absence of cross-linking in panel C indicates generation of a closed turnstile in module 3 following chain elongation (as represented by the “X” over the KS active site thiol), the appearance of a cross-linked adduct in panel F indicates that TE-catalyzed hydrolysis of the elongation product allows the reopening of the turnstile.