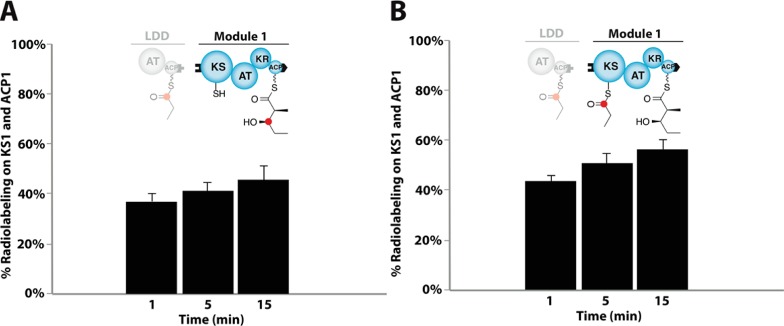
Figure 4.
Energetic coupling of the turnstile to polyketide chain elongation. In panel A, the ACP-bound diketide product of DEBS module 1 was generated by mixing holo-module 1 with DEBS loading didomain (LDD), 1-14C-propionyl-CoA, methylmalonyl-CoA, and NADPH. Consistent with data shown in Table 1B and Figure S6, radiolabel accumulated rapidly on module 1 and attained steady state corresponding to ∼50% occupancy of the ACP + KS sites. In contrast, in panel B the ACP domain of apo-module 1 was directly loaded with the formal diketide product of its chain elongation reaction. This was accomplished using the Coenzyme A thioester analogue of unlabeled diketide 1 in the presence of Sfp phosphopantetheinyl transferase. When the resulting module was mixed with LDD, 1-14C-propionyl-CoA, methylmalonyl-CoA, and NADPH, it was rapidly radiolabeled to a comparable steady state occupancy level, indicative of efficient translocation of 14C-propionyl units onto the KS domain of module 1. In both panels the occupancy values are reported as the mean ± SD (n = 3) of the ACP + KS occupancy for DEBS module 1. For experimental details, see the Supporting Information.