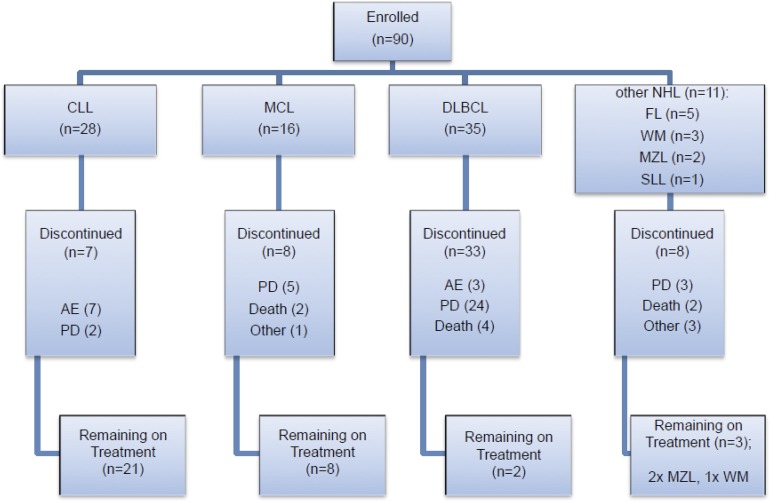
Figure 1.
Patient enrollment by disease subtype and disposition within the trial. Seven CLL patients were withdrawn due to AEs; 2 of the CLL patients withdrawn due to an AE also had concurrent PD. AEs were considered to be either related (R) or not related (NR) to study drug. In the CLL cohort, these AEs comprised: fever (NR), Escherichia coli septicemia (NR), worsening heart failure (NR), neutropenic sepsis (R), purpura, lymphocytic infiltration (R), spontaneous psoas hematoma (R), idiopathic thrombocytopenia (R). Three DLBCL patients were withdrawn due to AEs: myelodysplastic syndrome (NR), tubulopathy myeloma (renal tubular necrosis) (NR), and confusional state (NR). Two WM patients were withdrawn due to AEs: urticarial reaction (R), nonimmune drug reaction (R). Other reasons for discontinuation included investigator decision (2 DLBCL cases), patient decision (1 FL case), 1 case of MCL proceeding to allograft and DLTs in 2 WM patients. The majority of deaths especially in the DLBCL cohort were due to PD.