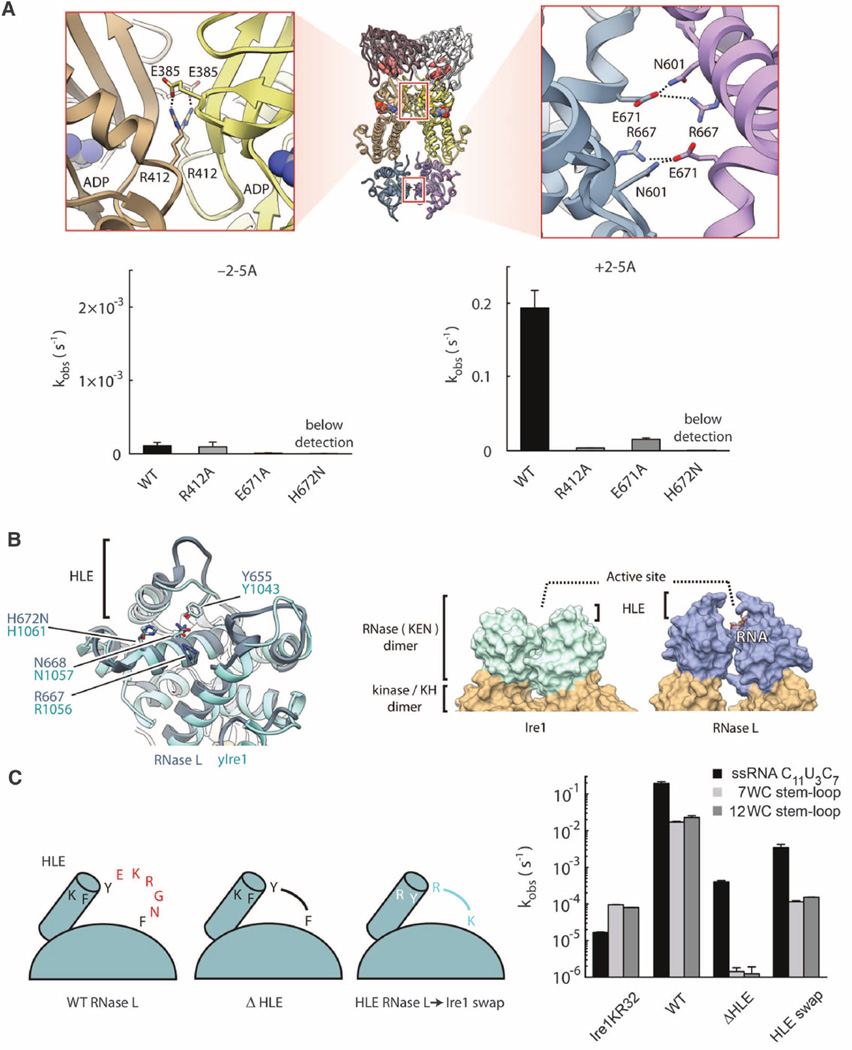
Fig. 2. KH and KEN homodimers.
(A) Symmetrical salt bridges stabilize the interfaces between the KH (1364 Å2) and RNase (943 Å2) domains. Mutations of these salt bridges impair RNase L activity. (B) Comparison of KEN domains in RNase L and Ire1. Key active-site residues are indicated. (C) Effect of HLE mutagenesis on cleavage of ssRNA and RNA hairpins derived from human XBP1 mRNA. WC indicates the number of Watson-Crick base pairs in the hairpin stem. Ire1KR32 is yeast Ire1 kinase/RNase. Reactions contained 100 nM Ire1KR32 and 5 nM RNase L. Error bars show mean ± SE.