Fig. 9.
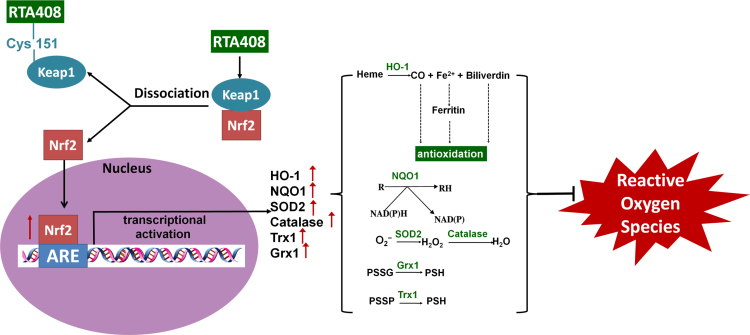
RTA 408 activates the Nrf2 pathway which leads to an upregulation of antioxidant enzymes and protects the cell from oxidative stress. Binding of RTA 408 to Cys151 in Keap1, the negative regulator of Nrf2, results in Keap1 inhibition. This promotes Nrf2 movement into the nucleus where it binds to the antioxidant response element (ARE). With the activation of ARE, transcriptional activation of antioxidant enzymes heme oxygenase-1 (HO-1), NADPH dehydrogenase (NQO1), superoxide dismutase 2 (SOD2), catalase, thioredoxin 1 (Trx1), and glutaredoxin 1 (Grx1) occurs. HO-1 converts heme to carbon monoxide, iron (II), and biliverdin, which all indirectly scavenge ROS. NQO1 converts enzymes and other proteins (R) back to their reduced form (RH) through the electron transfer between NADPH and NADP. Superoxide (O2−) can be converted to hydrogen peroxide using SOD2 and then further processed into water by catalase. Grx1 and Trx1 work together to reduce protein-glutathione mixed disulfide (PSSG) and protein-protein disulfide (PSSP) to protect protein thiols from oxidation. Overall, RTA 408 induction of phase II antioxidant enzymes such as HO-1, NQO1, SOD2, catalase, Grx1, and Trx1 via activation of Nrf2 promote RPE cell survival during oxidative stress.