Fig. 2.
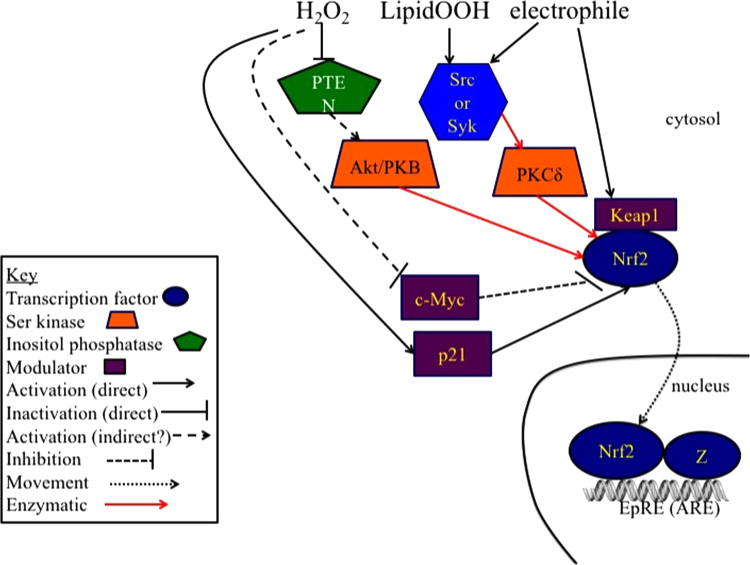
Electrophilic activation of Nrf2. When cysteines in Keap1 are modified by electrophiles, Nrf2 escapes Keap1 assisted ubiquitinylation and subsequent proteasomal degradation and can translocate to the nucleus [133], [134], [135]. Phosphorylation of Nrf2 by PKCδ and Akt assists in the nuclear translocation of Nrf2, where it increases transcription of genes through binding to the EpRE (also called ARE) element [136], [137]. PKCδ, and Akt are also activated by hydroperoxides and other electrophiles [138], [139], [140], [141], [142]. The proteins that are Nrf2 partners in binding to EpRE include Mafs G/F/K [143], c-Jun [144], c-Fos and Fra1 [145], c-Maf [146], JunD [147], [148], but little attention has been paid to whether any of their interactions with Nrf2 involve redox signaling. Similarly, little attention has been paid to the possibility of redox regulation of Bach1 and Nrf1 that compete with Nrf2 for binding to EpRE [149]. Furthermore, potential redox regulation of c-Myc inhibition of transcription through its binding to nuclear Nrf2 and acceleration of Nrf2 degradation [150] and the stabilizing influence of p21 [151] have yet to be explored in detail. All of these aspects may be part of the redox homeostasis involved with Nrf2. Additional redox-related activation of Nrf2 may occur during stress conditions through the sequestration of Keap1 by p62 such as occurs during dysregulation of autophagy [152].