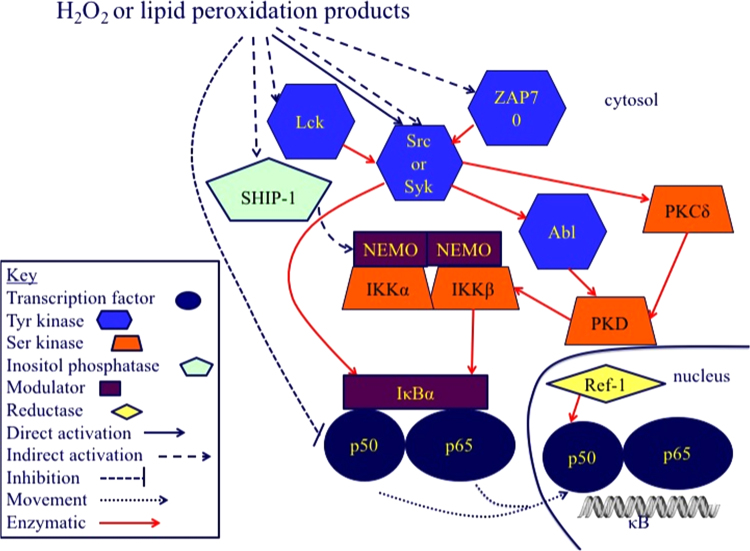
Fig. 3.
Hydroperoxide activation of NF-κB activation. NF-κB is composed of homo- and heterodimers of p50, p52, p65 (RelA), c-Rel, and RelB. Only p65, c-Rel, and RelB have transcriptional activation domains. IκB family members bind NF-κB dimers in the cytosol until IκB is phosphorylated and degraded. IκB isoforms differ among cell types in dependence upon the IKK complex for their phosphorylation. Two serine kinases, IKKα and IKKβ, and a modulator, NEMO (also called IKKγ) form the IKK complex that phosphorylates IκB. For an extensive description of NF-κB regulation not focusing on redox regulation, the reader is referred to some recent reviews [153], [154]. Early in the investigation of how H2O2 activated NF-κB, it became obvious that tyrosine phosphorylation was involved. H2O2, lipid hydroperoxides, and other electrophilic lipid peroxidation products, can directly activate members of the Src kinase family, Src, Syk, and Lyc and the closely related ZAP leading to activation of the tyrosine kinase Abl and serine kinase PKCδ that activate PKD. In an alternative pathway, Src or Syk directly phosphorylate IκB on a tyrosine residue, bypassing IKK activation. One of the more intriguing proposed targets in H2O2 activation of NF-κB is SHIP-1, an inositol-5-phosphatase. SHIP-1 activation by H2O2 has been shown to lead to the activation of the classical IKK-dependent pathway for NF-κB activation. SHIP-1 activity depends on its SH2 domain, which is of course, the classic target of tyrosine phosphorylation and in a study unrelated to NF-κB activation, SHIP-1 activity was demonstrated to be through its tyrosine phosphorylation by Lyn, another member of the Src kinase family [155]. Other possible pathways for NF-κB activation involving the Src kinases may exist; however, the activation of the Src kinases seems to be the key step through which most studies suggest H2O2 activates NF-κB. Although it is not clear exactly how and where NF-κB is oxidized, the redox chaperone activity of APE/Ref-1 is required to restore the ability of NF-κB to bind to DNA in the nucleus [156].