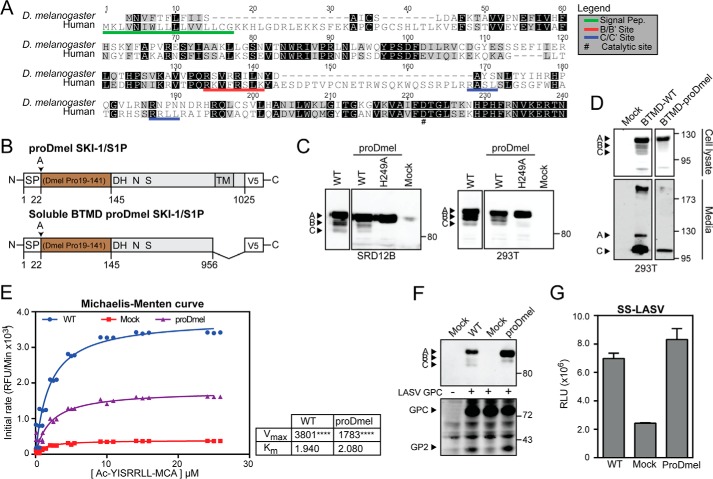
FIGURE 7.
The prodomain of D. melanogaster SKI-1/S1P is able to complement the human SKI-1/S1P form. A, alignment of the prodomains of human and D. melanogaster SKI-1/S1P. Alignment was performed with ClustalW running from the Geneious software package. Identical and similar residues are indicated by black and gray shading. Maturation sites are underlined in red and blue. B, schematic of the chimeric construct. The prodomain of D. melanogaster (aa 19–141) was fused to the human signal peptide (aa 1–22) and human SKI-1/S1P C terminus (aa 186–1052). The construct was tagged with a C-terminal V5 tag. C, maturation of chimeric and WT SKI-1/S1P. SRD12B and HEK293T cells were transfected with the indicated constructs and SKI-1/S1P detected in cell lysates by Western blot as in Fig. 1B. D, secretion of the SKI-1/S1P chimera. To verify that our chimeric construct underwent proper maturation and folding, a soluble BTMD form was generated. Constructs were expressed in HEK293T cells, and the presence of the SKI-1/S1P variants was detected in cell lysates and media. E, enzymatic activity of the chimera SKI-1/S1P proDmel-BTMD. Conditioned media from HEK293T cells expressing SKI-1/S1P proDmel-BTMD and WT were assayed for enzymatic activity as in Fig. 4C. Shown are Michaelis-Menten curves, Vmax, and Km values. Statistical significance between Mock and SKI-1/S1P forms Vmax was assessed by one-way analysis of variance with p < 0.001 (****) indicated. F, processing of LASV GPC by SKI-1/S1P proDmel-BTMD detected by Western blot as in Fig. 5C. G, activity of SKI-1/S1P proDmel against the SKI-1/S1P sensor was assessed by co-transfection into SRD12B cells. Conditioned media were analyzed for GLuc activity as in Fig. 5D. The data are shown as relative light units (RLU) (means ± S.D.; n = 3).