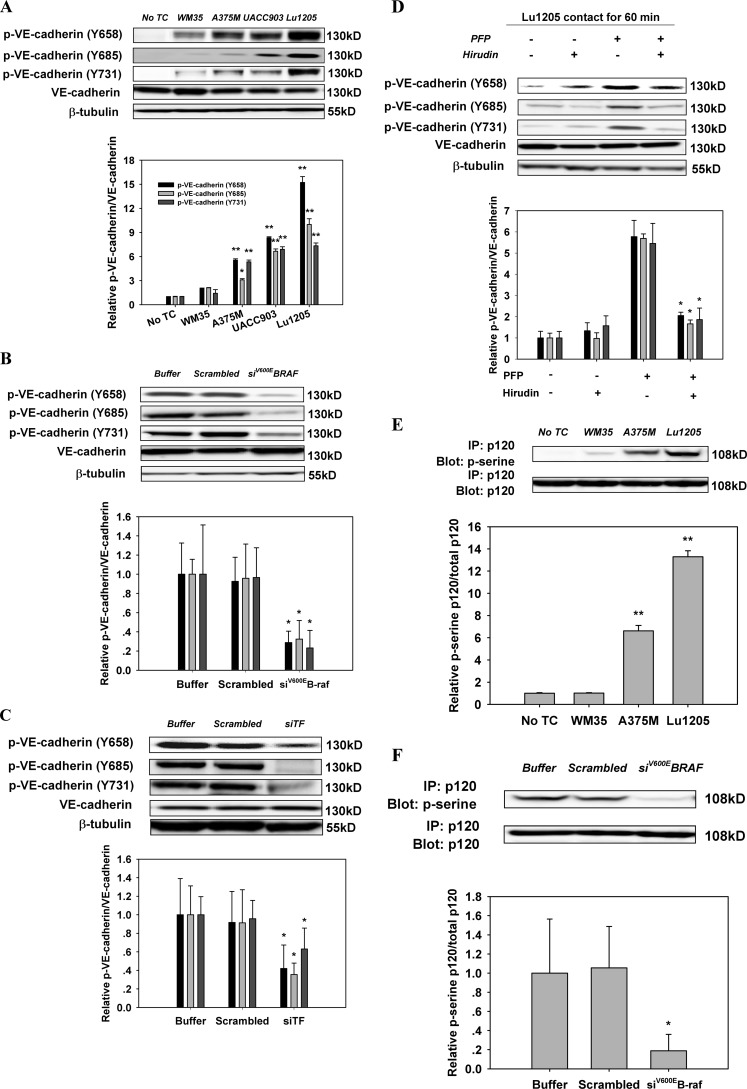
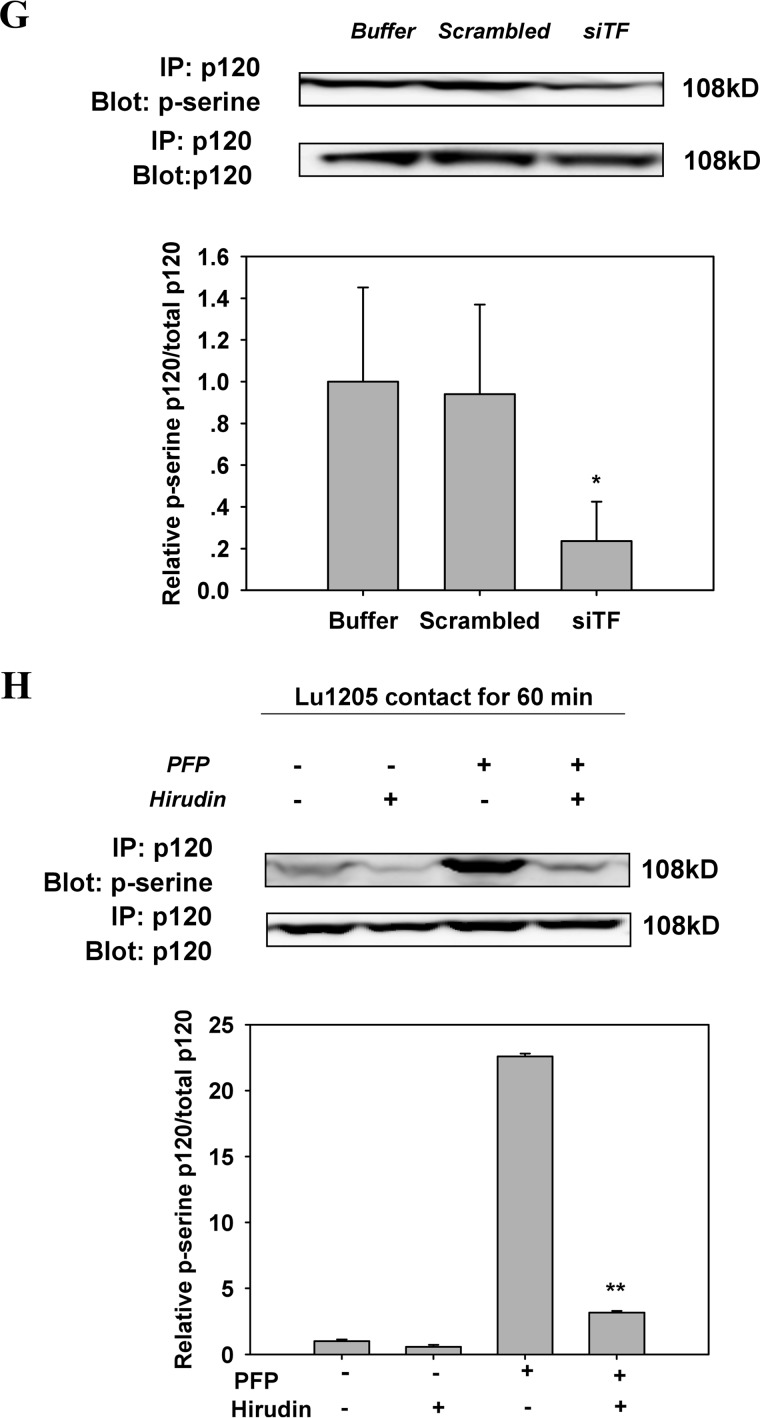
FIGURE 5.
Inhibition of the activities of B-Raf(V600E), TF, or thrombin reversed the increase of phosphorylation of VE-cadherin and p120-catenin in endothelium caused by melanoma contacts in plasma. A, co-culture between B-Raf(V600E)-positive melanoma and HUVECs promoted phosphorylation of VE-cadherin at tyrosine 658, 685, and 731. Confluent HUVEC monolayers were serum-starved for 12 h. They were either left alone (no TC) or co-cultured with 1 × 106 melanoma cells (WM35, A375M, UACC903, or Lu1205) in the presence of PFP for 60 min before Western blot analysis. B, silencing B-Raf(V600E) suppressed co-culture-induced tyrosine phosphorylation of VE-cadherin. HUVECs were subjected to Western blot analysis after being co-cultured with 1 × 106 Lu1205 melanoma cells transfected with buffer, scrambled siRNA, or B-Raf(V600E) siRNA in the presence of PFP for 60 min. C, silencing TF suppressed co-culture-induced tyrosine phosphorylation of VE-cadherin. HUVECs were subjected to Western blot analysis after being co-cultured with 1 × 106 Lu1205 melanoma cells transfected with buffer, scrambled siRNA, or TF siRNA in the presence of PFP for 60 min. D, hirudin suppressed co-culture-induced tyrosine phosphorylation of VE-cadherin. HUVECs were subjected to Western blot analysis after being co-cultured with 1 × 106 Lu1205 melanoma cells in the presence or absence of PFP with or without 40 units/ml hirudin for 60 min. E, serine phosphorylation of p120-catenin was triggered by melanoma contacts in the presence of PFP. HUVECs were left alone (no TC) or co-cultured with 1 × 106 melanoma cells (WM35, A375M, or Lu1205) in the presence of PFP for 60 min. HUVECs were subjected to immunoprecipitation (IP). F, serine phosphorylation of p120-catenin was suppressed by B-Raf(V600E) silencing. HUVECs were co-cultured with buffer, scrambled siRNA, or B-Raf(V600E) siRNA-transfected Lu1205 cells in the presence of PFP. HUVECs were subjected to immunoprecipitation. G, serine phosphorylation of p120-catenin was suppressed by TF silencing. HUVECs were co-cultured with buffer, scrambled siRNA, or TF siRNA-transfected Lu1205 cells in the presence of PFP. HUVECs were subjected to immunoprecipitation. H, serine phosphorylation of p120-catenin was abolished by hirudin treatment. HUVECs were subjected to immunoprecipitation after being co-cultured with 1 × 106 Lu1205 melanoma cells in the presence or absence of PFP with or without 40 units/ml hirudin for 60 min. For Western blot analysis, HUVECs were lysed and subjected to blotting using phospho-VE-cadherin-specific antibodies (Tyr(P)-658, Tyr(P)-685, and Tyr(P)-731). VE-cadherin and β-tubulin served as loading controls. For immunoprecipitation, precipitated proteins were analyzed by immunoblotting with an antibody against phosphoserine. The same blot was stripped and then reprobed with an antibody to p120-catenin. Relative VE-cadherin phosphorylation and p120 phosphorylation for densitometric analysis are shown below each blot. Values are mean ± S.E. *, p < 0.05; **, p < 0.01 compared with control. All results are representative of at least three independent experiments.