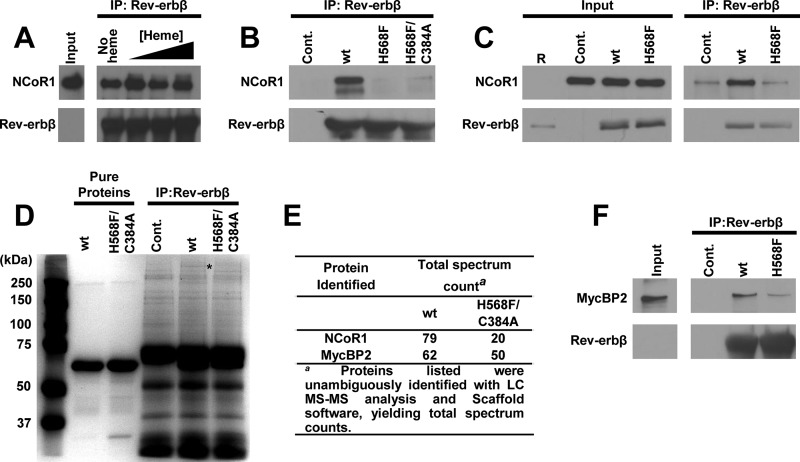
FIGURE 14.
Co-immunoprecipitation reveals heme-dependent interaction of Rev-erbβ with endogenous NCoR1 and MycBP2 from HEK293 cell extracts. A, co-IP of NCoR1 with FLRev-erbβHGS from soluble extracts depleted of heme. HEK293 cells were treated with 2 mm SA for 24 h and lysed, and a soluble extract was prepared. The heme content of those extracts was determined with an oxalic acid fluorescence assay, indicating control extracts contained 0.103 ± 0.005 nmol heme (mg protein)−1, whereas SA treatment lowered the heme content by 35% to 0.0669 ± 0.0003 nmol (mg protein)−1 (values represent the average of triplicate experiments ± S.D.). 1 mg of soluble extract was mixed with increasing concentrations of heme (denoted by a black triangle above the corresponding lanes; concentrations are 5, 10, and 20 μm) and 5 μg of recombinant FLRev-erbβHGS. Rev-erbβ LBD antibody was added to each sample, and the antibody-protein complexes were immunoprecipitated (IP), separated by SDS-PAGE, and electroblotted. Membranes were probed with NCoR1 C-20 (top panel) and Rev-erbβ QK-6 (bottom panel) antibodies. The input lane is 100 μg of cell extract (1/10th of the mass used in the IP) without recombinant protein added to demonstrate endogenous levels of NCoR1 and Rev-erbβ; the latter is undetectable under the exposure time necessary to observe the high levels of recombinant protein. B, a similar experiment as described for A, except the IPs did not contain heme, and recombinant FLRev-erbβHGS, H568F, and H568F/C384A were added to extracts. A control IP containing no recombinant protein was added to demonstrate the specificity of the NCoR1 interaction with the recombinant proteins (Cont.). C, HEK293FT cells were transiently transfected with pcDNA3.1(+) parent vector as a control, pcDNA3.1(+)-FLRev-erbβ for overexpression of wild-type Rev-erbβ (wt), or the analogous expression vector for the H568F variant. Rev-erbβ LBD antibody was added to the cell extracts, and the level of NCoR1 co-purifying with Rev-erbβ was assessed with immunoblotting. Input is 50 μg of extract (1/20th of the mass used in the IP) and Lane R = 50 ng of recombinant FLRev-erbβHGS. D, Coomassie-stained SDS-PAG demonstrating high molecular weight bands associated with wild-type FLRev-erbβMGC versus the H568F/C384A mutant. The asterisk indicates the location of the gel that was excised from the wild-type and H568F/C384A IP lanes, and those proteins within the gel slabs were identified with mass spectrometry. 5 μg each of pure FLRev-erbβMGC (wt) and H568F/C384A are shown for reference (denoted by black bars above the appropriate lanes). E, proteins with high total spectrum counts from the MS analysis are listed for both the wild-type and H568F/C384A samples. F, a similar IP as described in B, except the membrane was probed with a MycBP2 antibody showing a modest heme dependence to the MycBP2/Rev-erbβ interaction. The absence of MycBP2 in the control lane (Cont.) lacking recombinant protein indicates that the interaction of recombinant Rev-erbβ with the E3 ligase is specific.