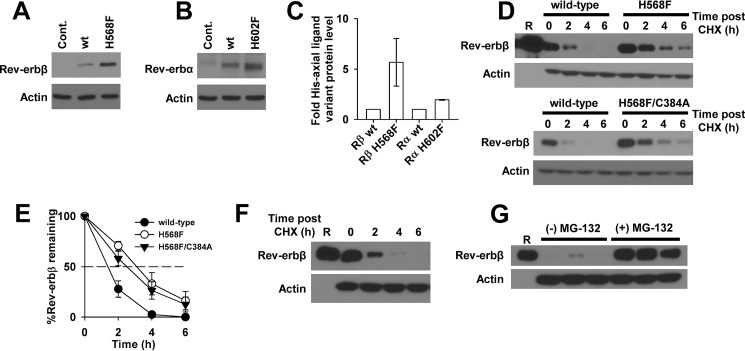
FIGURE 15.
Western blotting analysis indicates that heme induces Rev-erbβ protein degradation through a proteasome-dependent pathway. A, a Western blot demonstrating steady-state levels of Rev-erbβ in HEK293 cells transiently transfected with pcDNA3.1(+) parent vector (Cont., control), or the corresponding expression vectors for wild-type Rev-erbβ (wt) or the H568F variant (H568F). Cells (60–70% confluent in 35-mm-diameter plates) were transfected with 4 μg of vector and incubated for 24 h, and a soluble extract was prepared in radioimmune precipitation assay buffer. 30 μg of each extract was electroblotted and probed with Rev-erbβ QK-6 antibody (top panel) or actin as a loading control (bottom panel). B, same experiment as described for A, except cells were transfected with expression vectors for wild-type Rev-erbα (wt) or the H602F variant (H602F). Membranes were probed with Rev-erbα RS-14 antibody (top panel) or actin as a loading control (bottom panel). C, the intensities of the bands in A and B were quantified by densitometry, and the mean intensities ± S.D. of three experiments were plotted. For the Rev-erbα analysis, the low level intensity of the band in the control lane was subtracted from the intensities of the bands for wild-type and H602F Rev-erbα from the same experiment. D, HEK293 cells transiently transfected with pcDNA3.1(+)-FLRev-erbβ (wild-type) or the analogous expression vector for H568F (top experiment) or the H568F/C384A variant (bottom experiment) were treated with 20 μg ml−1 CHX. Cells were lysed and soluble extracts prepared at the indicated time points post-CHX addition. 50 μg of each extract was electroblotted and probed with Rev-erbβ QK-6 or an actin antibody as a loading control. Lane R, 25 ng of FLRev-erbβHGS. E, the intensities of the bands in D were quantified by densitometry, and the mean intensities ± S.D. of three experiments were plotted. F, a replicate CHX half-life experiment as described in D for cells overexpressing wild-type Rev-erbβ. Lane R, 5 ng of FLRev-erbβHGS. G, the same cells as described in F were treated with either 20 μg ml−1 CHX or 20 μg ml−1 CHX plus 20 μm MG-132 (denoted by black bars above the appropriate lanes; three biological replicates of each set are depicted). Cells were incubated for an additional 4 h prior to preparing extracts and blotting with Rev-erbβ QK-6, indicating that the proteasome degrades Rev-erbβ (i.e. a direct comparison can be made between time points from F and G, as these experiments were carried out on the same day).