FIGURE 4.
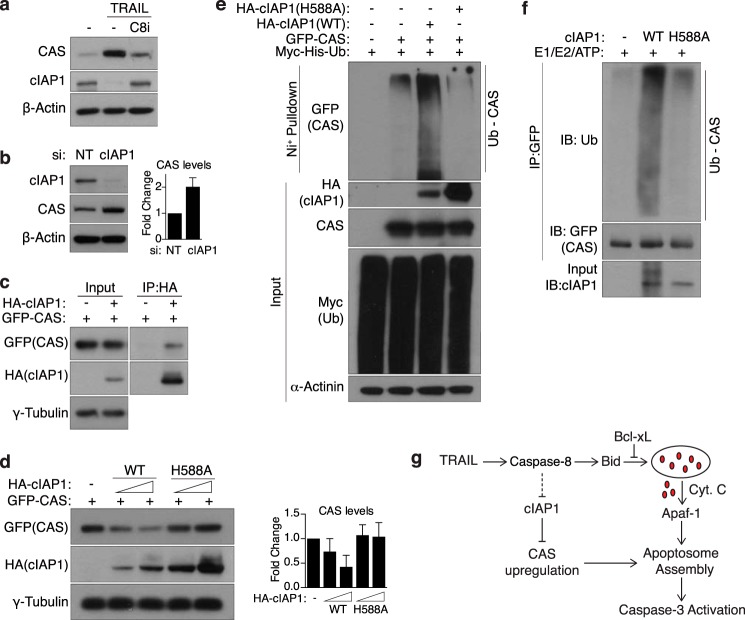
cIAP1 mediates the turnover of CAS and is degraded upon TRAIL treatment. a, MCF10A cells were treated with TRAIL (30 ng/ml, 4 h) and harvested for Western blot analysis. Where indicated, cells were pretreated with a caspase-8 inhibitor (C8i, 20 μm) for 30 min prior to addition of TRAIL. b, MCF10A cells were transfected with NT siRNA or siRNA against cIAP1 and harvested for Western blot analysis 72 h later. Right panel, quantification of CAS levels (mean ± S.D.) relative to NT siRNA cells from three independent experiments. c, lysates from 293T cells expressing GFP-CAS and empty vector or HA-cIAP1 were subjected to immunoprecipitation with HA-agarose beads. Bound proteins were then probed by Western blotting with the indicated antibodies. d, 293T cells were transfected with GFP-CAS and WT or E3-dead (H588A) cIAP1 and subjected to Western blot analysis 24 h later. The total amount of DNA transfected was balanced using empty vector DNA. Right panel, quantification of CAS levels (mean ± S.D.) relative to control cells from three independent experiments. e, HEK293T cells transfected with the indicated plasmids were treated with 25 μm MG132 for 4 h to allow for accumulation of ubiquitinated proteins. Whole cell lysates were then subjected to a nickel bead pulldown under denaturing conditions, and bound proteins were probed with the indicated antibodies. Note that, in d and e, expression of E3-dead cIAP1 is significantly higher than WT, presumably because of the lack of autoubiquitination-induced degradation. f, in vitro ubiquitination of GFP-CAS purified from 293T cells was performed using recombinant cIAP1, as described under “Experimental Procedures.” Note that WT-cIAP1 shows a smearing pattern because of autoubiquitination activity. IB, immunoblot. g, model illustrating the regulation of CAS. Upon stimulation by TRAIL, cIAP1 is degraded in a caspase-8-dependent manner, allowing for accumulation of CAS, which then feeds forward into the apoptotic pathway by stimulating apoptosome formation. Cyt C, cytochrome c.