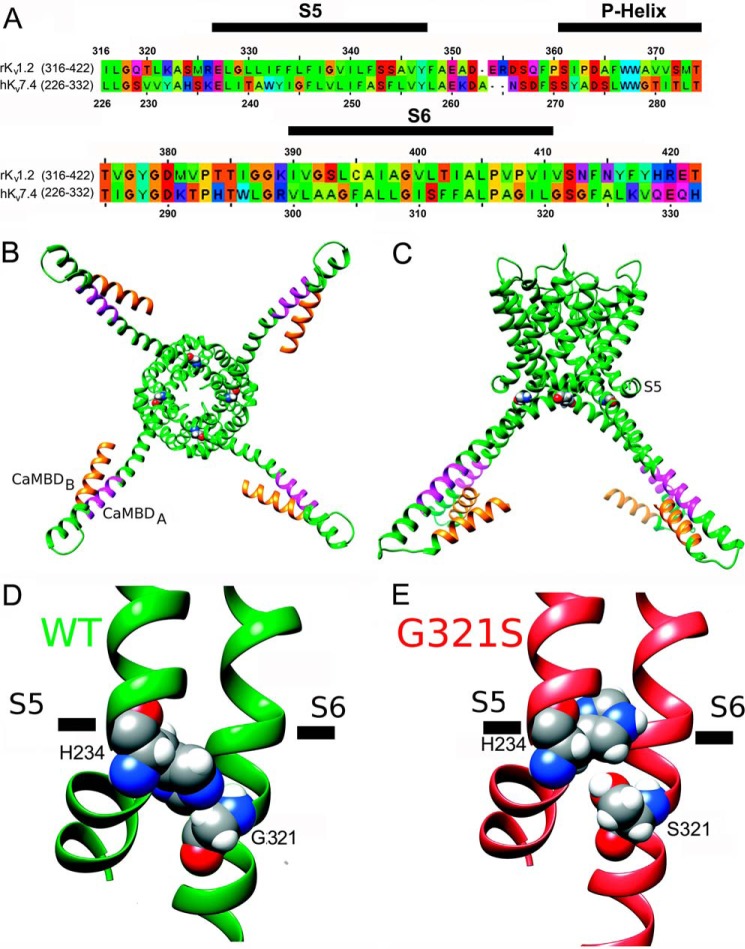
FIGURE 7.
Structural modeling of pore-forming domains and CaMBDs in the hKv7.4 WT and G321S MT. A, sequence alignment of the pore-forming domains of the hKv7.4 and rKv1.2 channels. The S5, P-helix, and S6 regions from the same subunit are marked by black bars above the sequence. B, structural model of the hKv7.4 WT viewed from the intracellular side of the membrane. The CaMBD A and B regions in the C terminus are colored orange and magenta, respectively. Gly-321 is shown in spacefilling representation. C, transmembrane view of the hKv7.4 model shown in B. D, close-up view of the structural model of the hKv7.4 WT. Gly-321 and His-234 are shown in spacefilling representation and labeled. E, close-up view of the structural model of the hKv7.4 G321S MT.