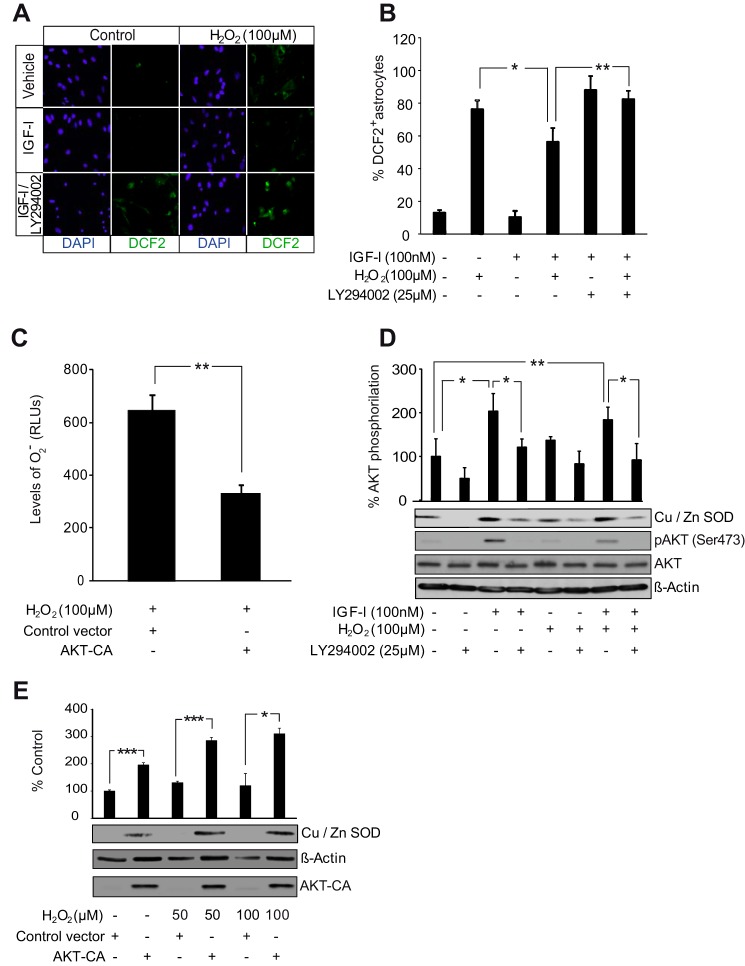
FIGURE 5.
AKT activation by IGF-I reduces ROS levels in astrocytes. A, representative photomicrographs of astrocytes stained with carboxy-H2DCFDA (DFC2) to detect ROS and with DAPI to stain cell nuclei. IGF-I (100 nm) lowered ROS levels after treatment of astrocytes with H2O2 (100 μm). Pretreatment with the PI3K/AKT inhibitor LY294002 (25 μm) prevented antioxidative effects of IGF-I. B, quantification of fluorocarboxy-H2DCFDA+ cells. IGF-I (100 nm) treatment markedly diminished the percentage of positive cells elicited by H2O2 (100 μm) (*, p < 0.05 versus H2O2-treated cells; n = 3). Pretreatment with LY294002 (25 μm) prevented the antioxidative effect of IGF-I (**, p < 0.01 versus IGF-I + H2O2-treated cells; n = 3). C, transfection with an AKT-CA construct significantly reduced levels (RLU, relative light arbitrary units) of O2˙̄ in H2O2 (100 μm)-treated astrocytes (**, p < 0.01 versus cells transfected with control construct; n = 3). D, IGF-I (100 nm) and its combination with H2O2 (100 μm) significantly up-regulated protein levels of the O2˙̄-detoxifying enzyme Cu,Zn-SOD (*, p < 0.05 and **, p < 0.01 versus control cells; n = 3). Pretreatment with LY294002 (25 μm) significantly prevented the IGF-I (alone or in combination with H2O2) antioxidative effect (*, p < 0.05 versus cells without LY294002 pretreatment; n = 3). AKT inhibition by LY294002 was monitored by detection of phospho-AKT (pAKT) (Ser-473) levels. β-Actin served as a loading control. E, AKT-CA astrocytes displayed significantly higher levels of Cu,Zn-SOD than control astrocytes both in normal conditions and in the presence of different doses of H2O2 (50 and100 μm) (***, p < 0.001 and *, p < 0.05 versus cells transfected with control construct; n = 3). AKT-CA expression was monitored using an anti-AKT antibody. β-Actin served as a loading control. Error bars represent S.E.